How is scanning probe electrochemistry used in battery research?
Latest updated: January 14, 2025Introduction
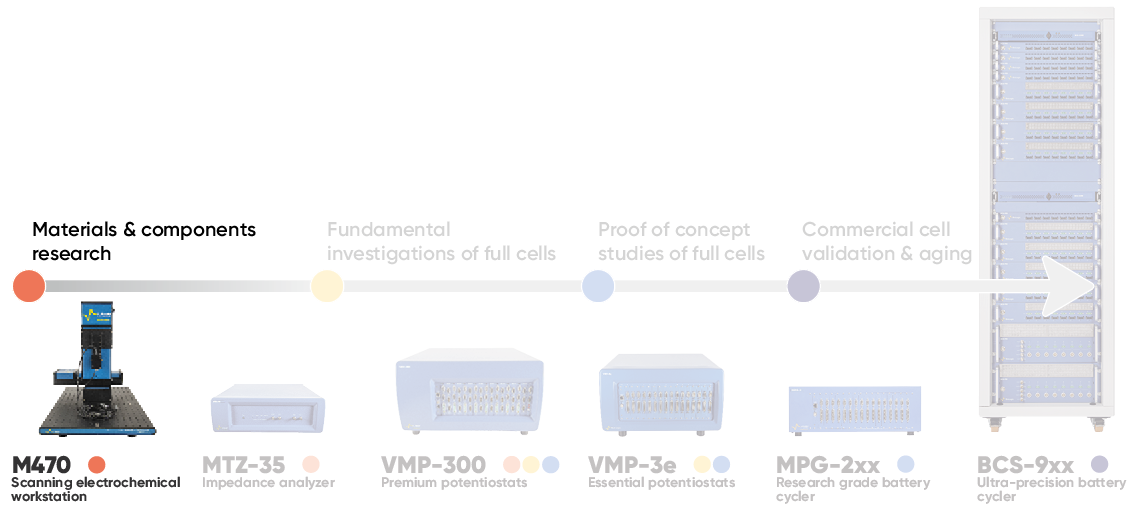
The growing push to improve battery technology requires a localized understanding of the system at the materials and components development stage, Fig. 1. One way to achieve this understanding is to utilize the family of techniques known as scanning probe electrochemistry, which includes, for example, Scanning Electrochemical Microscopy (SECM). In this article we explore why and how scanning probe electrochemistry is used in battery research.

Figure 1 : The M470 is used at the initial stage of battery research, when the materials and components are of interest.
Why is scanning probe electrochemistry used in battery research?
As batteries are based on materials subject to electrochemical processes, scanning probe electrochemistry is used in battery research because of the local information it provides, its non-destructive nature, and its ability to perform experiments in situ.
Local information
Although a lot of research on battery materials and components utilize bulk electrochemical studies, which assume a homogeneous sample, scanning probe electrochemistry is well suited to this work because of the local and heterogeneous nature of the electrochemical interfaces involved in battery materials. When efforts are made to understand the local behavior of a battery material using bulk electrochemical studies, further, sometimes difficult, analysis can be required to obtain local information. Because scanning probe electrochemistry techniques only measure the area under the probe, they provide true localized information on the battery sample, which when combined with raster scanning allows for a visual representation of the local differences in electrochemistry.
Non-destructive measurements
Further understanding of the Solid Electrolyte Interface (SEI) is important in the development of future batteries, especially those with novel chemistries, as a result, its formation and evolution is of great interest. Unfortunately, the SEI is very fragile, and electrically insulating, which can make it difficult to study. Scanning probe electrochemistry techniques are well suited to the study of the SEI because they offer a non-destructive local measurement of the sample. Furthermore, they are typically non-contact, avoiding damage to the SEI by the probe. By performing multiple measurements of the SEI sample at different times, or even biases, scanning probe electrochemistry provides a means to visualize changes to the SEI over time.
In-situ studies
Most scanning probe electrochemistry experiments, and especially those which have been applied to battery research, must be performed in electrolyte, this makes them inherently in situ techniques. This is an important trait for the study of battery systems as it aids in understanding the formation of the conductive or insulating layers.
How is scanning probe electrochemistry used in battery research?
SECM is by far the most used scanning probe electrochemistry technique used in battery research. However, Scanning Vibrating Electrode Technique (SVET), Local Electrochemical Impedance Spectroscopy (LEIS), and Scanning Droplet Cell (SDC) have all been used to a limited extent in battery research.
How is SECM used in battery research?
SECM has become the most popular option when scanning probe electrochemistry is used to study battery systems, due to its flexibility and resolution. Since it was first applied to battery research in 2007 [1], SECM has been used to look at everything from the battery electrodes to the SEI.
Study of battery electrodes by SECM
Because the SECM signal reflects the conductivity of the sample region beneath the probe, it is well suited to study the distribution of composite materials in a battery electrode and allows for comparison of this distribution between different electrodes [2]. By performing area maps in dc- or ac-SECM mode, users can directly visualize the heterogeneity of the electrodes of interest.
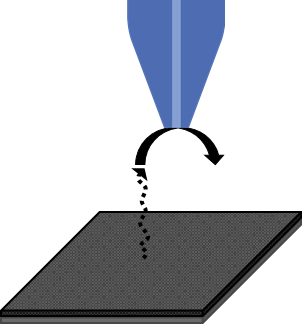
The changes of the battery electrode over time can also be studied with SECM. Owing to the high sensitivity of the probe to sample distance, SECM approach curves are able to investigate, in situ, the volumetric changes of battery electrodes over time due to electrode swelling, [3]. SECM can also be used to study the dissolution of species from the sample surface. This is accomplished through careful biasing of the SECM probe as it interacts with the dissolved species, in generator-collector mode. This has been demonstrated for Co2+ dissolution by Snook et. al. who used cyclic voltammograms at a probe in close proximity to a biased LiCoO2 electrode [4]. Fixing the probe at a set bias could allow dissolution of Co2+ or other redox active species to be mapped across the surface. Closely related is the study of the intercalation/deintercalation of Li from the battery electrodes. Because SECM is sensitive to changes in concentration of redox active species changes in the current measured at the probe will reflect Li intercalation (increase in current) and deintercalation (decrease in current) [5]. The use of SECM in generator collector mode to measure specific species related to battery materials, e.g. species dissolved from the electrodes, is illustrated in Fig. 2.

Figure 2 : SECM can be used in generator collector mode to measure species dissolved from the battery electrode, and even to investigate Li intercalation/deintercalation, as illustrated.
Study of the SEI by SECM
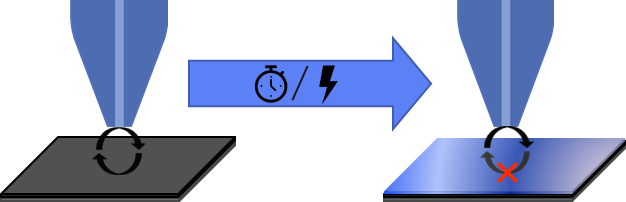
SECM is of particular use for the study of the formation and evolution of the SEI. Not only does SECM allow for local electrochemical information on the SEI, it also has a larger working probe to sample distance than other scanning probe electrochemistry techniques, making it particularly well suited to investigating this fragile layer. Both ac- and dc-SECM have use in studying the SEI. Typically, when the SEI is studied by dc-SECM the feedback mode is used [6], making area maps visually easy to interpret. In these studies regions with low current indicate the presence of the insulating SEI, while regions with high current indicate regions with exposed electrode. ac-SECM area maps are also visually easy to interpret, with heterogeneity in the impedance of area map, reflecting the heterogeneity of the SEI across the surface [7]. It is possible to repeat these area scans over the same area after a given period, or a certain number of cycles to visualize changes to the SEI, this could be growth, or it could be degradation due to instability, bubble formation, or local damage [8]. This use of SECM to investigate SEI growth is illustrated in Fig. 3.

Figure 3 : As illustrated SECM can be used to study the formation of the SEI with time or changing bias. The SEI formation results in a change in feedback mechanisms from positive feedback to negative feedback.
Study of solid electrolytes by SECM
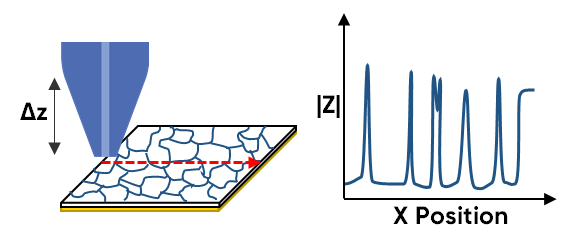
SECM has been used to study solid electrolytes, of interest for All Solid-State Batteries (ASSB), providing insight on the grains and grain boundaries. This is an application Intermittent Contact (ic)-ac-SECM is particularly well suited for, because of the underlying topography of these solid electrolytes, which would otherwise distort the impedance measured by the ac-SECM measurement. Using ic-ac-SECM the local impedance is measured, which reflects conductivity, with low impedance modulus indicating a conductive region. The resulting local impedance maps, therefore, allow for the local conductivity of the grains and grain boundaries to be compared visually [9, 10]. This use of ic-ac-SECM is illustrated in Fig. 4. While it is possible to use bulk EIS to gain an understanding of the grains and grain boundaries, it is an average of their response, and even with what is typically complex analysis to understand the contributions of the grains and grain boundaries, bulk EIS does not provide information on local variations in the contributions of these two feature types.

Figure 4 : ic-ac-SECM has been used to investigate the relationship between the impedance of grains and grain boundaries in solid electrolytes as illustrated.
How is SVET used in battery research?
Although the use of SVET in battery research is limited, it was the first scanning probe electrochemistry technique to be demonstrated in this field, back in 1994 by Ishikawa et. al. [11]. In this work SVET was used to study the effects of different organic additives on the interface formed on a Ni electrode, by Li deposition during application of a discharge voltage. Measurements of the local ionic current of the sample allowed comparison between the heterogeneity, and current magnitude of the electrodes in the presence of different additives. Since this research SVET has also been used to study the effect of applying different biases to a model electrode, allowing the effect of reduction and oxidation to be followed in situ [12].
How is LEIS used in battery research?
There has also been limited use of LEIS in battery research, in this case to directly measure the local impedance of battery materials. Typically, the LEIS measurements performed have been used to compare bare and coated battery materials [13-15]. The change in heterogeneity of the LEIS impedance map is of particular interest in understanding the effect of the coating on the battery electrode.
How is SDC used in battery research?
As of yet the application of SDC in battery research has been limited to proof-of-concept studies. These studies demonstrate, however, the potential for SDC in battery research to form and evaluate the SEI [16], study the efficiency of Li plating and stripping [17], and even its possibility as a means of performing high throughput studies on battery materials [18]. The potential of SDC in battery research rests in its ability to perform direct local electrochemical experiments, like cyclic voltammetry, on the sample. Because the electrochemical cell in SDC is merely a three-electrode cell shrunk down to the size of a droplet, results from these measurements can be straightforward to interpret.
Conclusion
Scanning probe electrochemistry has been introduced as a family of techniques of interest in understanding the local properties of battery materials and components to further advancements in this field. SECM has been shown to be particularly useful in this field because of the flexibility of properties it can be used to measure. Although it has become the most used scanning probe electrochemistry technique in battery research, other techniques in the scanning probe electrochemistry family have also been used, including SVET, LEIS, and SDC.
To learn more about selecting the best scanning probe electrochemistry technique for your application, in battery research or otherwise, please see the article: Which scanning probe electrochemistry technique?
References
- J. Liu, Y. Yang, F. Gao, W. Chen, Y. Li, P. Yu, H. Shao, Electrochimica Acta 52 (2007) 4231-4238
- L. Wang, L. M. Housel, D. C. Bock, A. Abraham, M. R. Dunkin, A. H. McCarthy, Q. Wu, A. Kiss, J. Thieme, E. S. Takeuchi, A. C. Marschilok, K. J. Takeuchi, ACS Applied Material Interfaces 11 (2019) 19920-19932
- H. Bülter, F. Peters, J. Schwenzel, G. Wittstock, J. Electrochem. Soc. 163 (2016) A27-A34
- G. A. Snook, T. D. Huynh, A. F. Hollenkamp, A. S. Best, Journal of Electroanalytical Chemistry 687 (2012) 30–3
- G. Zampardi, E. Ventosa, F. La Mantia, W. Schuhmann, Chemical Communications 49 (2013) 9347-9349
- S. Liu, D. Liu, S. Wang, X. Cai, K. Qian, F. Kangabe, B. Li, Journal of Materials Chemistry A 7 (2019) 12993-12996
- D. Liu, Q. Yu, S. Liu, K. Qian, S. Wang, W. Sun, X.-Q. Yang, F. Kang, B. Li, Journal of Physical Chemistry C 123 (2019) 12797-12806
- H. Bülter, F. Peters, J. Schwenzel, G. Wittstock, Angewandte Chemie International Edition 53 (2014) 10531-10535
- S. Catarelli et al., Frontiers in Energy Research 4 (2016) 14
- T. Takami, Y. Morita, M. Yonemura, Y. Ishikawa, S. Tanaka, M. Mori, T. Fukunaga, E. Matsubara, ACS Applied Energy Materials 6 (2018) 2546-2554
- M. Ishikawa, S. Yoshitake, M. Morita, Y. Matsuda, J. Electrochem. Soc. 141 (1994) L159-L161
- L. Wang, Q. Wu, A. Abraham, P. J. West, L. M. Housel, G. Singh, N. Sadique, C. D. Quilty, D. Wu, E. S. Takeuchi, A. C. Marschilok, K. J. Takeuchi, Journal of The Electrochemical Society 166 (2019) A3575-A3584
- S.-K. Cho, H.-I. Kim, J.-W. An, K. Jung, H. Bae, J. H. Kim, T. Yim, S.-Y. Lee, Advanced Functional Materials 30 (2020) 2000792
- D. Lee, H.-I. Kim, W.-Y. Kim, S.-K. Cho, K. Baek, K. Jeong, D. B. Ahn, S. Park, S. J. Kang, S.-Y. Lee, Advanced Functional Materials 31 (2021) 210385
- S.-Y. Lee, S.-H. Kim, M. Kim, I. Kristanto, W.-Y. Kim, K. Ryu, H.-I. Kim, K. Y. Ma, C. Heo, S. K. Kwak, Y. S. Meng, H. S. Shin, 06/12/2023, Horizontal lithium electrodeposition on atomically polarized monolayer hexagonal boron nitride, https://doi.org/10.21203/rs.3.rs-3636934/v1
- D. Muñoz-Torrero, C. Santana Santos, E. García-Quismondo, S. Dieckhöfer, T. Erichsen, J. Palma, W. Schuhmann, E. Ventosa, RSC Adv. 13 (2023) 15521
- S. Dieckhöfer, W. Schuhmann, E. Ventosa, ChemElectroChem 8 (2021) 3143–3149
- A. Sanin, H. S. Stein, 15/07/2023, Exploring reproducible non-aqueous scanning droplet cell electrochemistry in model battery chemistries, https://doi.org/10.26434/chemrxiv-2023-t39wm