How to decode the standard test methods for corrosion?
Latest updated: October 8, 2024Introduction
Corrosion standards were developed to provide appropriate procedures for carrying out corrosion tests on specified metallic materials and alloys. The corrosion technique section in EC-Lab® software includes 6 techniques in compliance with ASTM and JIS standard procedures.
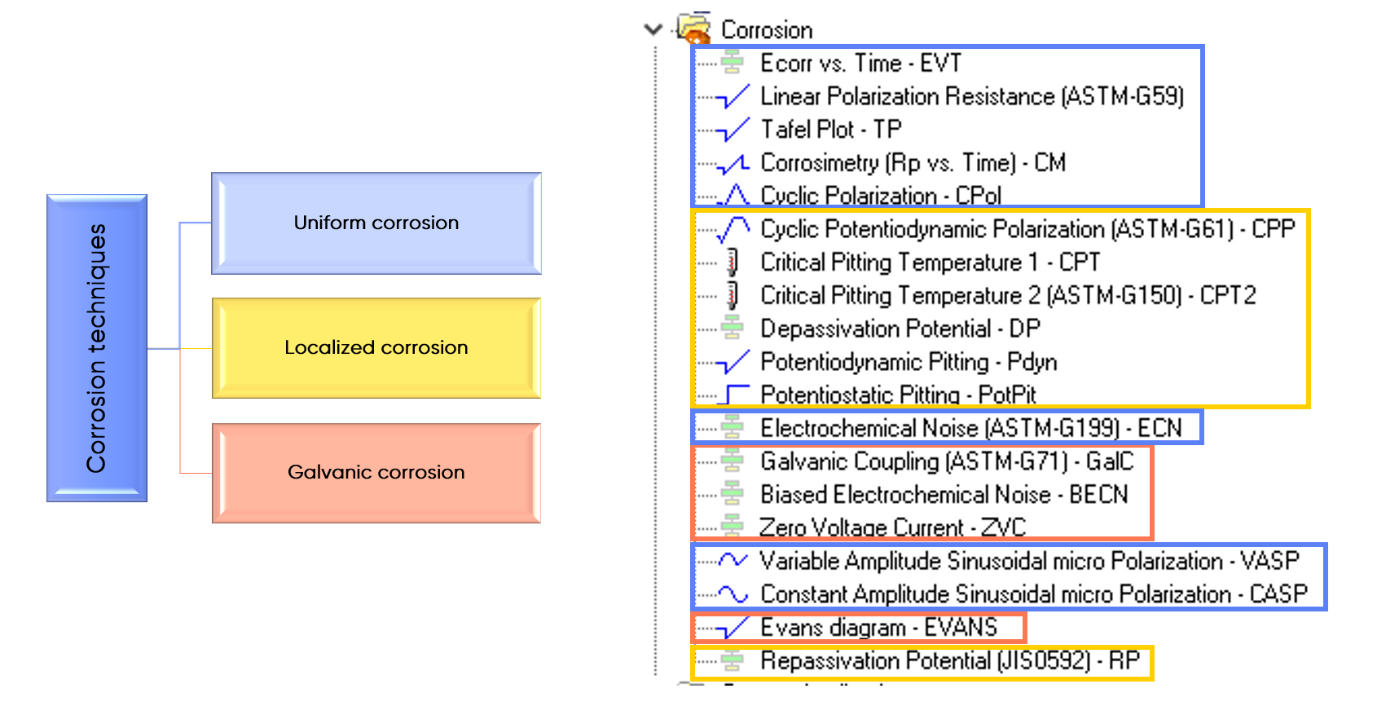
Uniform corrosion can be investigated with Linear Polarization Resistance technique (ASTM-G59), Electrochemical Noise technique (ASTM-G199).
Galvanic corrosion can be investigated with Galvanic coupling technique (ASTM-G71).
Localized corrosion can be investigated with Cyclic Potentiodynamic Polarization technique (ASTM-G61), Critical Pitting Temperature technique (ASTM-G150) and Repassivation Potential technique (JIS 0592).
Here’s a tip: With EC-Lab software, you can create your own protocol and save it in the technique list, thanks to Custom Applications , to run the experiment whenever you need.
Want to learn more about corrosion theory? Check out our article about corrosion basics.
Uniform corrosion
ASTM–G59: Linear Polarization Resistance (LPR)
The linear polarization resistance technique is used in corrosion monitoring. This technique is especially designed for the determination of a polarization resistance of a material and corrosion current through potential steps around the corrosion potential.
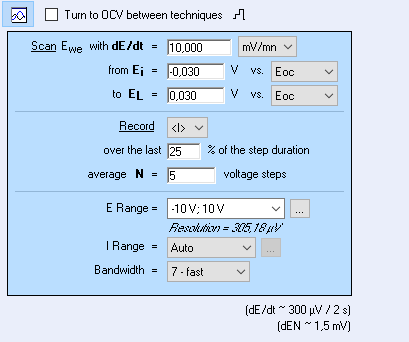
In EC-Lab® software, the LPR technique allows the user to measure the polarization resistance of a corroding system following the ASTM – G59 standard procedure.
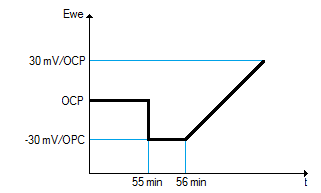
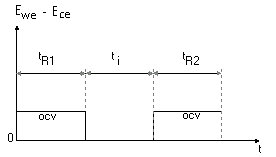
In this procedure, the Open Circuit Potential (OCP or Eoc) of the sample is measured at 5 and 55 minutes to ensure the system stationarity. Then the sample is polarized at -30 mV vs Eoc for 1 minute, after which the potential is scanned from -30 mV to 30 mV vs Eoc at a speed of 10 mV/min.
Figure 1 : Linear Polarization Resistance technique (ASTM-G59)
In EC-Lab® software, auto range is available to optimize the current measurement (ensure maximum accuracy) during the voltage sweep. Averaging of current over a chosen percentage of the step duration enables smoothing of data and measurement of mainly faradaic current.
The $R_\text p$ Fit tools available in EC-Lab® software can be used to determine the polarization resistance ($R_\text p$) and corrosion current ($I_\text{corr}$). It can also be used to determine the corrosion rate.
Here’s a tip: Corrosimetry technique available in EC-Lab® is an advanced procedure designed to follow automatically standard values ($R_\text p$, $E_\text{corr}$, $I_\text{corr}$) evolution versus time for several months
ASTM-G199: Electrochemical Noise (ECN)
The Electrochemical Noise is a technique for the measurement of voltage and current fluctuations occurring when two electrodes are shorted: the Electrochemical Current Noise (ECN) and the Electrochemical Potential Noise (EPN).
In most cases, the coupling current is measured between two electrodes from the same material. The microstructure differences between these two electrodes results in the fact that one of them behaves anodically and the other one behaves cathodically.
In EC-Lab® software, the ECN technique allows the user to measure the electrochemical noise following the ASTM – G59 standard procedure.
The procedure consists of applying zero volts between the working electrode and the counter electrode and then measuring the current and the potentials of both the working and counter electrode versus the reference electrode.
Figure 2 Electrochemical Noise technique (ASTM-G199)
The fluctuations are of very small amplitude, and this is why the choice of the instrument used in this purpose is critical. Check out our series of articles explaining why BioLogic instruments are capable of performing reliable noise measurement on electrochemical systems according to recommendations provided by ASTM.
Premium range instruments are recommended for ECN measurements, as use of the analog filter is required, refer to our article giving guidelines for proper ECN measurement for more details. The quality of the measurement will be greatly enhanced if the cell is placed in a Faraday cage, in order to remove environmental noise and other electrodynamics perturbations.
Electrochemical Noise Analysis (ENA) tools available in EC-Lab® enable the determination of noise resistance (and polarization resistance).
Galvanic corrosion
ASTM-G71 : Galvanic Coupling (GalC)
ASTM-G71 covers conducting and evaluating galvanic corrosion tests to characterize the behavior of two dissimilar metals in electrical contact in an electrolyte under low-flow conditions.
In EC-Lab® software, GalC technique follows the ASTM – G71 standard procedure. It consists of applying zero volts between the working electrode and the counter electrode and then measuring the current and the potentials versus the reference electrode.
Figure 3 Galvanic Coupling technique (ASTM-G71)
This technique is a direct measurement of the galvanic current. The current data can then be converted into a theoretical corrosion rate based on Faraday’s law.
Localized corrosion
ASTM–G61: Cyclic Potentiodynamic Polarization (CPP)
The Cyclic Potentiodynamic Polarization (CPP) is the most common electrochemical test for localized corrosion resistance. The cyclic potentiodynamic polarization measurement is used for the determination of the relative susceptibility to localized corrosion of passivable materials.
In EC-Lab® software, the CPP technique allows the user to measure the pitting susceptibility of a system following the ASTM – G61 standard procedure.
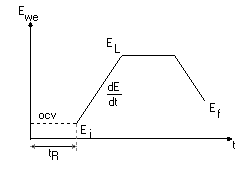
This technique consists of applying a voltage sweep with a current limit ($I_\text p$) on the measurement current. When the measured current reaches the current $I_\text p$ (or the corresponding set current density), the forward voltage sweep stops, and the reverse voltage sweep starts.
Figure 4 Cyclic Potentiodynamic Polarization technique (ASTM-G61)
The obtained current vs potential plot allows the user to determine the corrosion potential, the corrosion current, the passive current, the pitting potential and the re-passivation potential.
ASTM–G150: Critical Pitting Temperature (CPT2)
Critical Pitting Temperature technique is used for the determination of the temperature at which initiation of localized corrosion occurs. It is used for the evaluation of stainless steel and related alloys to pitting corrosion.
In EC-Lab® software, the CPT2 technique enables the user to measure the lowest temperature at which stable propagating pitting occurs on the tested sample, following the ASTM – G150 standard procedure.
This technique consists of a temperature regulation phase and a polarization phase. After an initial temperature stabilization period of a minimum of 10 min, the sample is heated at a rate of 1°C/min. As explained in the ASTM-G150 standard, the sample is anodically polarized to a potential above the pitting potential range. This potential is held constant during the whole temperature scan. A potential of 700 mV versus SCE (25°C) is suitable for most stainless steels. The current is monitored during the temperature scan, and the CPT is defined as the temperature at which the current increases rapidly, which for practical reasons is defined as the temperature at which the current density exceeds 100 μA/cm2 for 60 s. The experiment stops when a pitting current is reached.
The resulting data is a series of current/time curves at different temperatures, allowing the user to determine the critical pitting breakdown temperature of the system.
Figure 5 Critical Pitting Temperature technique (ASTM-G150)
Note: In EC-Lab® software, the user can find CPT1 and CPT2 (ASTM-G150) techniques. The critical pitting temperature is measured using a temperature ramp protocol in the CPT2 technique whereas a temperature steps protocol is used in the CPT1 technique.
In EC-Lab® software, “External devices” parameters enables users to set the external thermostat for the control of the temperature.
Looking for an electrochemical cell for corrosion? The Avesta cell was developed for pitting corrosion testing in compliance with ASTM-G150.
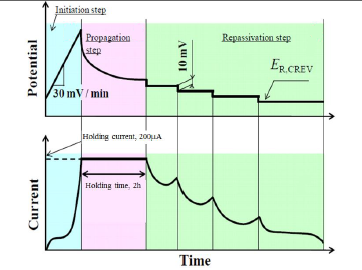
JIS 0592: Repassivation potential (RP)
The Japanese Industrial Standard (JIS) specifies a method for the determination of the passivation potential for crevice corrosion from the reciprocating anode polarization test of stainless steels in a neutral aqueous solution containing chloride ions.
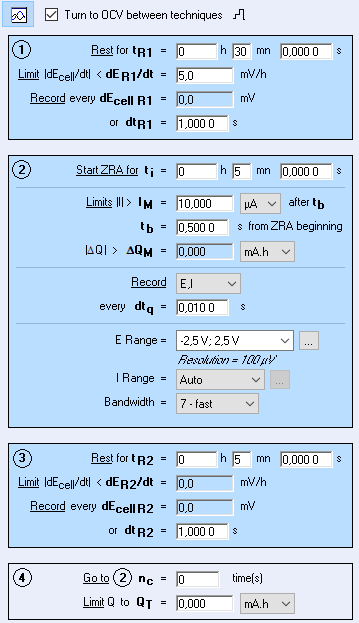
In EC-Lab® software, the Repassivation potential technique allows the user to measure the passivation potential following the JIS 0592 standard procedure. This method includes a potentiodynamic, galvanostatic and potentiostatic steps, respectively. Other sequences can be added to these steps.
Figure 6 Repassivation potential technique ( JIS 0592)
Conclusion
BioLogic instruments are able to perform reliable measurement in compliance with ASTM and JIS standard procedure. Currently, 6 techniques following these standards are available in the corrosion section of EC-Lab® software.