Rotating Ring Disk Electrode and Scanning Electrochemical Microscopy: Two Related Techniques
Latest updated: January 14, 2025Introduction
For continuous improvement in energy storage and conversion solutions, e.g. batteries and fuel cells, research into the materials and components is required. Two bipotentiostat techniques which can offer insight at this early stage of research are Rotating Ring Disk Electrode (RRDE) and Scanning Electrochemical Microscopy (SECM), Fig. 1. Although just two options in a toolkit of techniques used in energy storage and conversion research RRDE and SECM provide related information, therefore it is not uncommon to see them used to study the same systems, sometimes even in the same study. Both RRDE and SECM have been used to investigate catalysts used in fuel cells and electrolyzers [1], as well as to study batteries, for example to look at the Solid Electrolyte Interface (SEI) [2,3], and the decomposition of battery electrodes [4, 5].

Figure 1 : RRDE and SECM are used in the first stages of energy storage and conversion, during the study of materials and components.
RRDE is an evolution of the Rotating Disk Electrode (RDE) technique. The RRDE is made of two electrodes, a central disk electrode, which is surrounded by a ring electrode. The disk and ring electrode are separated by an insulator and are biased independently by a bipotentiostat. During the RRDE experiment the electrodes are rotated, which pulls product from the disk to the ring, resulting in a generator collector type experiment [6], illustrated in Fig. 2. Using RRDE it is possible to investigate kinetics parameters, such as the rate constant. For more information on the RRDE please see: Rotating Ring Disk Electrode: an Introduction.

Figure 2 : The RRDE experiment is illustrated.
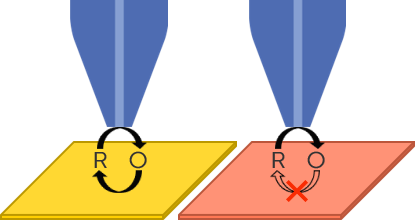
SECM uses a biased probe near to a sample to measure its local electrochemistry. This is possible because when in proximity, the sample influences the signal measured at the probe. Samples with high electrochemical activity, e.g. conductors, will result in a high signal at the probe, while samples with low to no activity, e.g. insulators, will result in a low signal measured at the probe. This is illustrated in Fig. 3. For more information on SECM please see: SECM101: An Introduction to Scanning Electrochemical Microscopy.

Figure 3 : SECM when the probe is over a conductor (left) and insulator (right) is illustrated.
How are RRDE and SECM related?
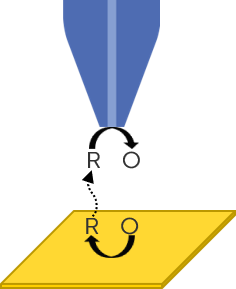
Although SECM has been used in its many modes in studies alongside RRDE and RDE, it is most analogous to RRDE when used in the Generator-Collector (GC) mode, with Sample Generator/Tip Collector (SG/TC) being the most common mode. In SG/TC the sample generates the redox species of interest, either naturally or through the application of a bias, which diffuses to the probe, which is biased to collect it, as illustrated in Fig. 4. While in RRDE it is the rotation of the electrode driving the diffusion and convection of the sample from disk to ring, in SECM it is the very small gap between probe and sample, resulting in a thin film cell, which allows for the fast diffusion between sample and probe.

Figure 4 : The SECM Generator-Collector mode is illustrated.
In RRDE each experiment is performed on an individual sample to obtain bulk information about that sample, which means ideally the electrodes must be homogeneous and flat. Because the SECM probe can be scanned around the sample surface, however, it can obtain local information about the sample, allowing for the study of heterogeneous samples, and even arrays of samples in a single experiment. These samples may be flat, or there could be some surface roughness.
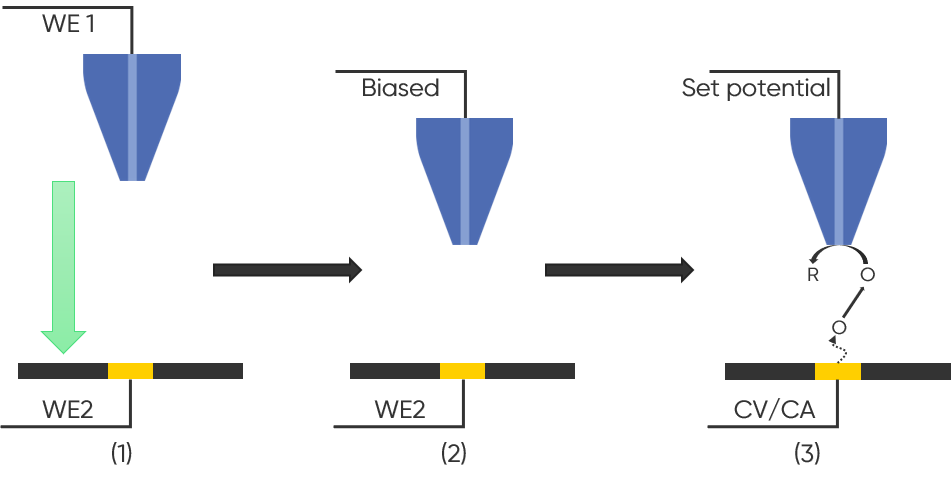
As a bulk electrochemical experiment, RRDE is used to quantitatively study the sample to determine kinetics parameters. SECM, however, is often used in a qualitative manner, with the probe raster scanned across the sample surface to map sample activity. Using SG/TC mode an SECM map will show the highest activity regions as regions with the highest signal (typically current) magnitude, allowing for easy visualization of sample activity. SG/TC can also be used for quantitative studies, with, for example Tip Substrate Voltammetry (TSV)-SECM being analogous to RRDE. In this mode the probe is approached to the sample, and a bias is applied to the probe. While the probe is biased at a set potential throughout, a Cyclic Voltammogram (CV) or ChronoAmperommetry (CA) experiment is performed at the sample [7]. As a result, any species produced at the sample are detected by the probe, with the resulting plot being the probe current as a result of sample potential, allowing for quantitative analysis. TSV-SECM is illustrated in Fig. 5.

Figure 5 : TSV-SECM is illustrated. In step (1) the probe is approached to the sample; the probe is biased in step (2) and finally in step (3) a CV or CA is run at the sample resulting in the probe detecting species generated by the sample.
The comparison between RRDE and SECM is summarized in Table 1, which includes some general information about the setup of each.
Table 1: Comparison of RRDE and SECM.
| Rotating Ring Disk Electrode (RRDE) | Scanning Electrochemical Microscopy (SECM) |
|---|---|
| Experiment | |
| Standard electrochemical technique | Advanced electrochemical technique |
| Diffusion from sample to ring occurs because of rotation | Diffusion from sample to probe occurs because of small probe to sample gap |
| Used to study individual samples | Used to study individual samples, or arrays of samples |
| Bulk information | Local information |
| Sample should be homogeneous | Sample can be homogeneous or heterogeneous |
| Sample should be flat for best analysis | Sample can be flat or rough |
| Quantitative studies | Qualitative and quantitative studies |
| Setup | |
| Available using BluRev | Available using SECM470 |
| Controlled using EC-Lab software | Controlled using M470 software |
| Coupled with bipotentiostat – Typically SP-300 | |
| ac- and dc- modes available | |
| 4-electrode cell | 3- or 4- electrode cell |
Conclusion
SECM and RRDE can be used together to build a complete picture of the sample of interest. They provide related data allowing for an understanding of the bulk and local nature of a sample. As a result, the use of both techniques in a study is not uncommon. For further information on the use of either technique for your application, please contact your local BioLogic representative.
References
- N. Limani, A. Boudet, E. Scorsone, V. Derycke, B. Jousselme, R. Cornut, Electrochem. Comm. 153 (2023) 107538
- P. Schwager, H. Bülter, I. Plettenberg, G. Wittstock, Energy Technol. 4 (2016) 1472-1485
- S. Liu, D. Liu, S. Wang, X. Cai, K. Qian, F. Kangabe, B. Li, Journal of Materials Chemistry A 7 (2019) 12993-12996
- S. E. Lee, O. C. Harris, T. Siboonruang, M. Tang, Joule 5 (2021) 551-563
- H. Bülter, F. Peters, J. Schwenzel, G. Wittstock, J. Electrochem. Soc. 163 (2016) A27-A34
- C. G. Zoski, Handbook of Electrochemistry, Elsevier, Amsterdam, (2007)
- A. Papaderakis, A. G. Anastopoulos, S. Sotiropoulos, J. Electroanal. Chem. 783 (2016) 217-225