SDC or SECM for high throughput scanning?
Latest updated: November 19, 2024Introduction:
With local electrochemical techniques there can be multiple options to measure analogous data, requiring researchers to carefully consider the most suitable technique for their application. Two such techniques are Scanning Electrochemical Microscopy (SECM) and Scanning Droplet Cell (SDC). Both techniques offer researchers the ability to measure the local current (or voltage if used galvanostatically), or impedance, Fig 1. As a result, understanding how the two techniques compare is important.
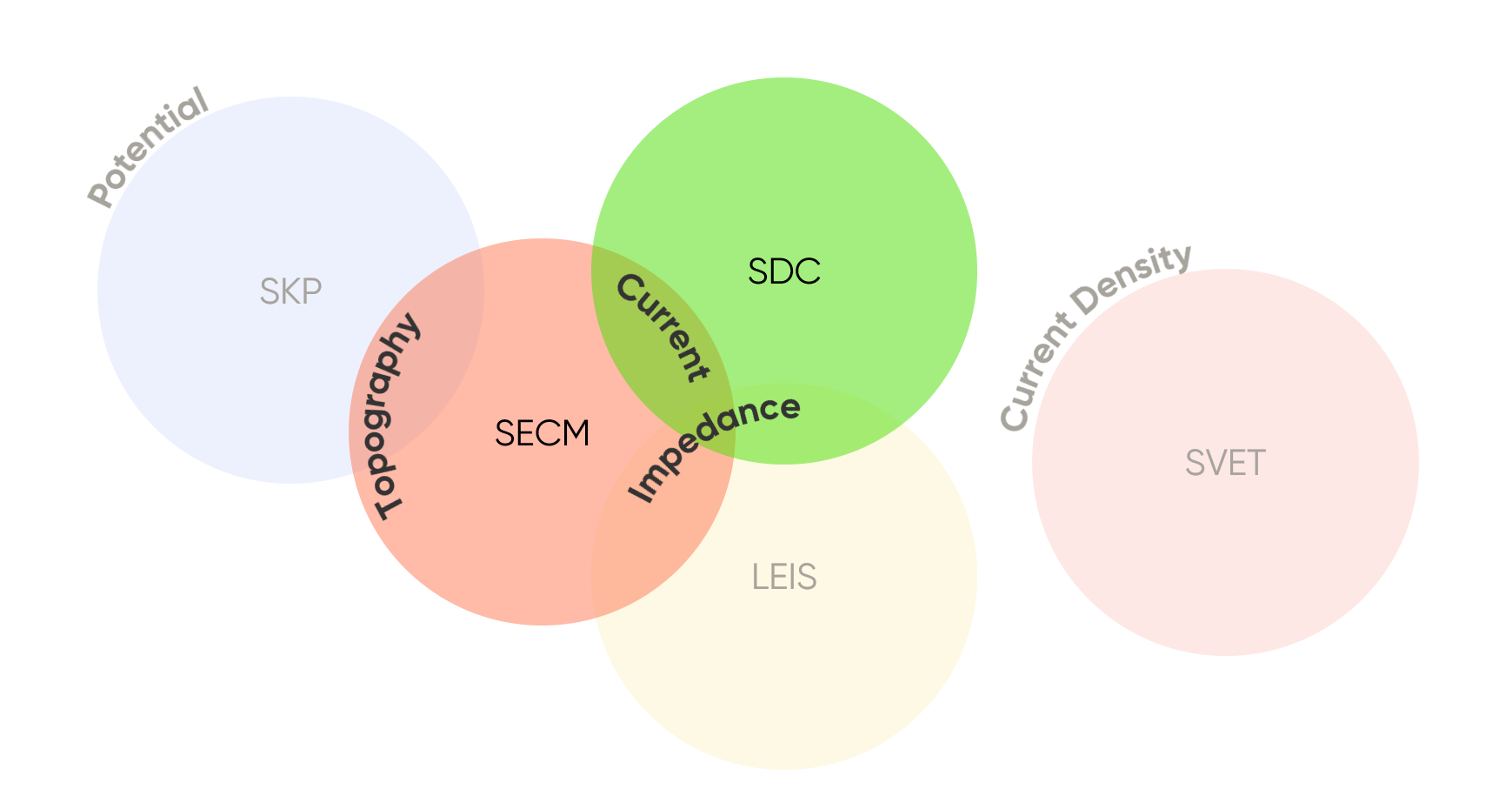
Figure 1: Comparison of the signals measured by the different local electrochemistry techniques, with a focus on SECM and SDC.
In SECM a biased probe is held in close proximity to the sample of interest, allowing the sample to influence the signal measured at the probe. SECM can be used in both the dc- and ac- modes depending on the exact signal of interest. In the most common mode of the dc-SECM technique, feedback mode, the sample is immersed in a solution with a redox mediator present, with the probe biased potentiostatically to interact with this mediator. When the biased probe is near a conductive region of the sample, there is an increase in current compared to the current measured by the probe in bulk solution. On the other hand, if the probe is near an insulating region of the sample, there is a decrease in current compared to the current measured by the probe in bulk solution. Through this feedback mechanism the activity of a sample can be mapped. dc-SECM is illustrated in Fig. 2, with more information on the technique found in the article: SECM101: An Introduction to Scanning Electrochemical Microscopy. When working with ac-SECM an ac-bias is applied to the probe in close proximity to the sample. Although the mechanism is more complex than in dc-SECM (and outside the scope of this article), in ac-SECM the sample also influences the signal measured at the probe allowing local activity to be mapped. More information on ac-SECM can be found in the article: ac-SECM101: An Introduction to Alternating Current-Scanning Electrochemical Microscopy.
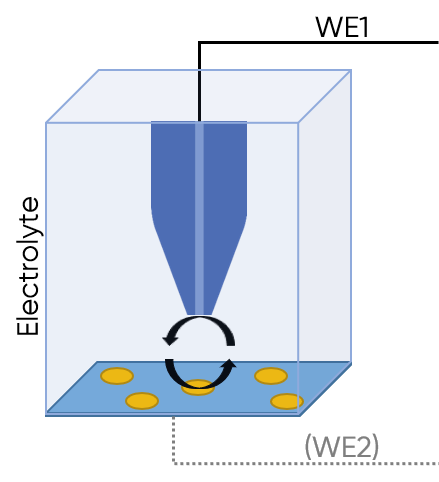
Figure 2: dc-SECM is illustrated. The probe acts as the main working electrode and is in close proximity to a sample in electrolyte. The sample may be connected as the second working electrode, but this is not required. Positive feedback over a conductive region is illustrated.
SDC utilizes a droplet in contact with the sample to perform local electrochemistry investigations. A specialized head is used which contains the reference and counter electrode, as well as acting as the electrolyte source for the droplet. The electrolyte may be held in a reservoir in the SDC head (static head), or it may be continually pumped through the SDC head (flow head), in turn refreshing the electrolyte droplet during use. The sample is connected as the working electrode, with only the area under the droplet addressed in each local measurement. Because of the flexibility of the SDC setup it is possible to use it in ac- and dc- mode, for area/line scans, and for local electrochemistry and impedance measurements. When using the SDC flow head it can also be used to investigate the effect of electrolyte flow on the electrochemical reaction occurring at the sample. For more information on SDC please see SDC101: An Introduction to Scanning Droplet Cell.
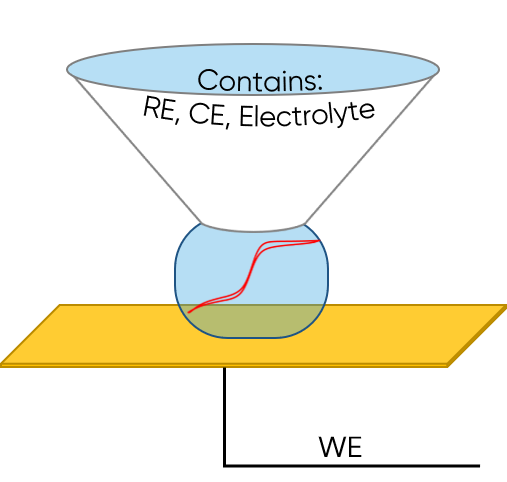
Figure 3: SDC is illustrated. The sample is the working electrode. The SDC head contains the reference and counter electrode, and electrolyte. When the SDC head is in close proximity to the sample a droplet forms.
SECM vs. SDC
SECM and SDC can be used to measure similar information about a sample, specifically by measuring a current signal, in dc-mode, or the sample impedance when used in ac-mode. As a result, it is not uncommon for both techniques to be considered in certain fields, for example corrosion, and catalysis, including catalysts for fuel cells and electrolyzers, and CO2 electrolysis, especially when high throughput screening is considered. For these fields both techniques can be considered, requiring a comparison to determine which is most applicable to a given investigation.
There are several key differences between SECM and SDC which should be considered when deciding between them. The resolution of the two techniques is commonly a deciding factor when selecting between SECM and SDC. In SECM the resolution of the measurement is determined by the probe diameter, which typically runs from submicron to tens of µm. For SDC the resolution is determined by the droplet size, which itself is dependent on the sample and electrolyte used, typically this is hundreds of µm. The reverse of this, however, is that SDC is typically used to measure over a larger area than SECM.
The electrochemical connection of the cell should also be considered. In SECM the cell is typically in a 3-electrode configuration, with the probe acting as the working electrode. In more limited cases, it is used in a 4-electrode configuration, with both the probe and sample acting as separate working electrodes (as seen in Fig. 2). In most SECM experiments the sample is not the working electrode, and it does not need an electrical connection made to it. This has the added advantage that the SECM sample can be fully conductive, fully insulating, and anything in between, including a matrix of isolated conductive regions in insulating regions.
SDC uses a 3-electrode cell, with the sample acting as the only working electrode, requiring an electrical connection to it. As a result, SDC requires the sample to be a conductor or semiconductor. If the sample has insulating regions, it can still be measured, however no signal will be measured in these regions as the 3-electrode cell is broken.
The requirements for the sample in SECM and SDC differ in other ways; in SECM the probe is an electrode, and the entire sample is exposed to electrolyte, while in SDC the probe is a droplet in contact with the sample, exposing only a small amount of the sample to electrolyte at any one time. SECM can be used to measure impermeable and porous samples, while SDC samples should not be porous because the droplet will be distorted. SECM can be used when measuring hydrophobic or hydrophilic samples, while for SDC the samples are ideally hydrophilic, as hydrophobic samples can cause difficulties in forming a good droplet. SECM can be used in the investigation of smooth samples, when measured in constant height mode, or it can be used to measure rough samples, or samples with background topography, when using Intermittent Contact (ic)-SECM to perform constant distance measurements. SDC, on the other hand, is typically used in constant height mode to measure smooth samples. Finally, although both SECM and SDC can be used in ac- or dc-mode, to perform area scans or local electrochemistry and EIS experiments across a surface, it is important to consider how both are typically used. The typical use of SECM is to perform area scans of the sample generating visual maps of local activity, while for SDC the typical use is instead to perform local electrochemistry experiments at key locations across a surface.
Or both?
Although it can be a choice of either SECM or SDC, that is not always the case. In those scenarios where both SECM and SDC are of interest the M470 can be equipped with both techniques allowing users the flexibility to choose for a given experiment.
Conclusion:
SECM and SDC are both techniques that can be used for either ac- or dc-experiments and are suitable options for performing high throughput screening, especially in the fields of corrosion and catalysis, including catalysts for fuel cells, electrolyzers, and CO2 electrolysis. Knowing which technique is best suited requires examining several factors such as sample material, resolution needed and desired application. Table 1 summarizes the difference between the two techniques.
Table 1: Table comparing SECM and SDC.
| SECM | SDC |
|---|---|
| Resolution | |
| Resolution determined by probe diameter | Resolution determined by droplet size |
| Sub to 10s of µm resolution | 100s to 1000s of µm resolution |
| Smaller scan area | Larger scan area |
| Electrochemical cell | |
| 3- or 4-electrode cell | 3-electrode cell |
| 3-electrode cell: Probe is the working electrode1 4-electrode cell: Probe and sample are the working electrodes |
Sample is the working electrode |
| Electrical contact to the sample is not required | Electrical contact to the sample is required |
| Electrochemical measurements performed at the probe | Electrochemical measurements performed at the sample |
| Entire sample is exposed to electrolyte | Only the sample area under the droplet is exposed to the electrolyte |
| Samples | |
| Fully conducting to fully insulating | Fully conducting and semiconducting |
| Impermeable and porous | Impermeable |
| Hydrophobic and hydrophilic | Ideally hydrophobic |
| Smooth flat, rough or with topography2 | Smooth flat |
| Entire sample is exposed to electrolyte | Only the sample area under the droplet is exposed to the electrolyte |
| Measurement | |
| Constant height or constant distance measurement2 | Constant height measurement |
| Electrolyte is static throughout the measurement | Electrolyte can be refreshed in the droplet throughout the measurement |
| Probe is positioned near the sample using approach curve | SDC head is visually positioned near the sample |
| Smaller scan area | Larger scan area |
| ac- and dc-experiments available | ac- and dc-experiments available |
| Application | |
| Can be used for area scans, or local electrochemistry and EIS experiments across the sample. | Can be used for area scans, or local electrochemistry and EIS experiments across the sample. |
| Typical use is to perform area scans across a sample. | Typical use is to perform local electrochemistry or EIS experiments across the sample. |
| Wide range of fields of use including catalysis, corrosion, biochemistry, batteries, sensors, and more | Fields of use are typically catalysis (including fuel cells and CO2 electrolysis) and corrosion |
| Applied to high throughput screening | Applied to high throughput screening |
| M470 Module | |
| Available using SECM470 module | Available using SDS470 module |
1There are limited cases where the sample may be the working electrode
2When ic-SECM470 is used
Related products




