SDC101: An Introduction to Scanning Droplet Cell
Latest updated: November 18, 2024What is Scanning Droplet Cell?
Scanning Droplet Cell (SDC) is a scanning probe electrochemistry technique in which the probe is a droplet confined to the surface of a conducting or semi-conducting sample. In Scanning Droplet Cell, the droplet probe acts as the electrochemical cell to map the electrochemical properties of a sample, or perform truly local standard electrochemistry measurements at the sample of interest.
Scanning Droplet Cell was originally introduced in 1997 by Hassel et. al. [1] to locally investigate oxide layers on metal surfaces. It has since been extended from these original corrosion-based studies, to studies in catalysis, sensors, fuel cells and more.
How does Scanning Droplet Cell work?
Scanning Droplet Cell is an extension of the electrochemical droplet cell technique. In this technique the three-electrode cell exists in a droplet confined to the surface of the sample, which acts as the working electrode. It is therefore a requirement of the technique that the sample is a conductor or semi-conductor. Improving on the electrochemical droplet technique, Scanning Droplet Cell also allows the droplet to be raster scanned across the sample to build a map of local activity.
To allow the electrochemical cell to be confined to a droplet Scanning Droplet Cell uses a special head as the probe, which contains both the counter and reference electrode, and the electrolyte of interest. When the SDC head is near the sample surface contact can be made between the sample and the droplet completing the electrochemical cell. The droplet is maintained by the surface tension between the tip of the SDC head, and the sample surface. The resolution of the Scanning Droplet Cell experiment is ultimately determined by the size of the droplet. With droplet size determined by the aperture and aspect ratio of the hole in the SDC head, the electrolyte used, and the hydrophobic nature of the sample.
There are two options for Scanning Droplet Cell measurements. These measurements can be performed with scanning, or whilst stationary. In scanning measurements, a dc bias, or a single ac frequency, is applied to the sample while the probe is scanned across the surface to create a line or area map. The other option is for the probe to be held stationary and traditional electrochemistry experiments, or Electrochemical Impedance Spectroscopy (EIS) are performed at the area under the droplet. To compare the electrochemical activity of different features the droplet can be moved between features and the electrochemical, or EIS measurement repeated. This provides direct local measurements of the sample.
What are the components of a Scanning Droplet Cell instrument?

In Fig. 1 the components of an instrument capable of performing Scanning Droplet Cell measurements are annotated.
As with other instruments used to perform scanning probe electrochemistry experiments the control electronics act as an interface between the software and all of the electronic components of the system. The x,y,z scanning stage allows the SDC head to be approached to the sample surface to form the interface between the droplet and the sample, and to automatically raster scan the surface in x and y. Scanning Droplet Cell measurements use a combined potentiostat and Frequency Response Analyzer (FRA). The potentiostat allows a dc bias to be applied to the sample, and for a full suite of electrochemistry experiments to be run. On the other hand, the inclusion of an FRA allows ac-SDC experiments to be performed. This includes mapping measurements performed at a single ac frequency, and single point measurements performed over a range of frequencies.
The final component is the SDC head which acts as the probe in Scanning Droplet Cell measurements. The SDC head acts as the source of the electrolyte for the droplet, while also housing the reference and counter electrodes. There are two types of SDC heads which may be used in a Scanning Droplet Cell measurement: (1) a reservoir head and (2) a flow head. When a reservoir head is used all of the electrolyte available to the droplet is housed within the SDC head. The electrolyte remains the same throughout the measurement. When a flow type SDC head is used, however, a pump is attached to the SDC head to flow the electrolyte through the head past the counter and reference electrodes, and the sample, Fig. 2. In this case the electrolyte can continually be replenished throughout the measurement. This is particularly useful when the electrochemical process under study produces a lot of product which could otherwise be detrimental to the final measurement, for example through contamination of the electrolyte. A final consideration with regards to the SDC head is the aperture and aspect ratio of the head opening. While it is ultimately the droplet which determines the final measurement resolution this is in part dependent on the aperture and aspect ratio of the head opening of the SDC head used.
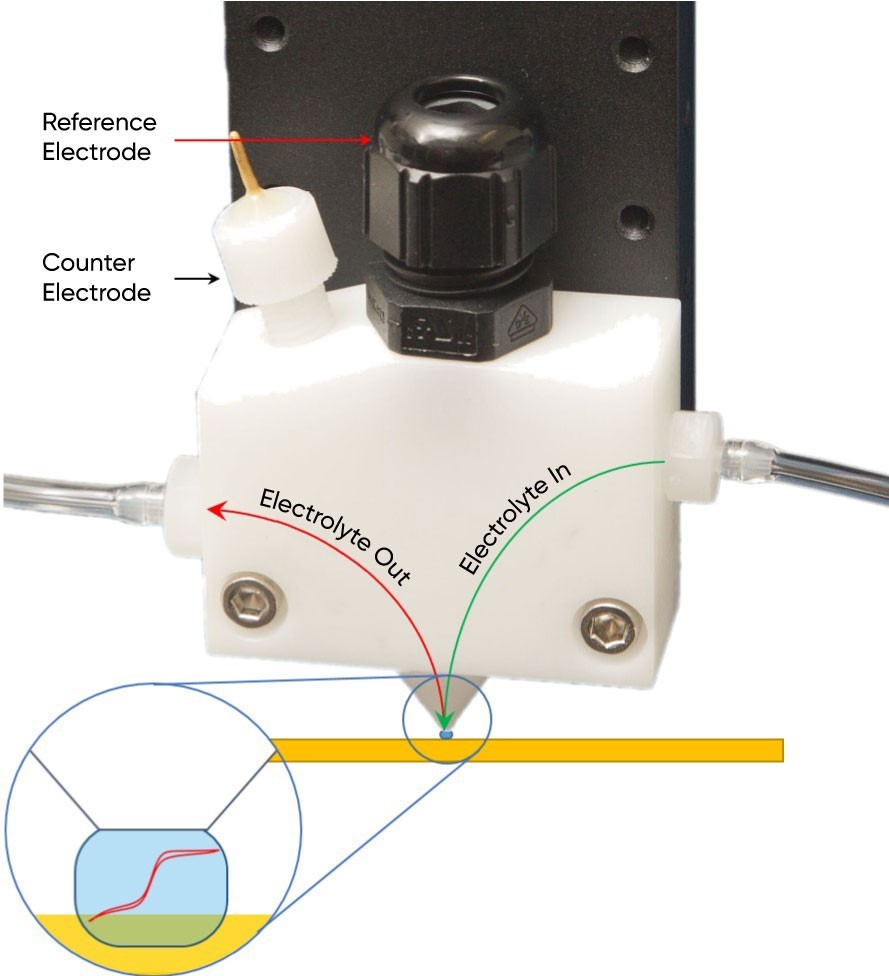
Figure 2: A flow type SDC head is shown, with the flow of electrolyte in and out marked. The droplet has been enhanced for illustration purposes.
Why use Scanning Droplet Cell?
One of the main advantages of Scanning Droplet Cell is that the electrolyte is confined on the sample only to the area under the droplet. This is important when full exposure of the sample to electrolyte is undesirable. This is the case when the sample may be evolving through electrolyte exposure, such as in corrosion, and risks being in a different state after the duration of an area scan. Furthermore, by confining the electrolyte when standard electrochemistry experiments, like cyclic voltammetry, are performed on the working electrode sample these are local measurements, rather than bulk measurements, which provide only an average of the sample electrochemistry. A further result of this is the fact that Scanning Droplet Cell is a direct measurement of the local electrochemical properties of the sample, unlike some other scanning probe electrochemistry techniques. This means standard electrochemical theories can be applied directly to the results measured.
What is Scanning Droplet Cell used for?
Figure 3: The uses for Scanning Droplet Cell
Scanning Droplet Cell measurements can be applied to any conducting or semi-conducting sample for which the local electrochemical characteristics are of interest, as such it has been applied to a wide range of fields, Fig. 3. Specific uses of Scanning Droplet Cell include:
• Investigating the variation of corrosion properties across and away from a sample feature [2]
• Studying the effect of doping on the electrical conductivity of a donor/acceptor material [3]
• Screening catalyst compositions for the production of H2 fuel [4]
• High throughput screening of catalyst combinatorial libraries [5]
• Examining the effects of grains and grain boundaries on the electrochemical properties of a material [6]
The applications of Scanning Droplet Cell, and other scanning probe electrochemistry techniques have been covered in detail in a series of Learning Center articles focusing on the questions they can answer in each field. The most recent article addresses the questions which can be answered by scanning probe electrochemistry in fuel cell research.
Further Information
BioLogic offers a wide variety of tutorials, application notes, and technical notes to expand your understanding of the techniques we offer. And keep checking back on our Learning Center, which we update regularly!
Glossary
Flow head: An SDC head in which the electrolyte of interest is flowed through the head to continually refresh the electrolyte of the droplet.
Reservoir head: An SDC head in which the electrolyte available for the droplet is held within the head. The electrolyte remains the same throughout the measurement.
References
- W. Hassel, M. M. Lohrengel, Electrochim. Acta 42 (1997) 3327-3333
- M. Thuss, J. R. Kish J, J. R. McDermid, in: Mathaudhu S.N., Sillekens W.H., Neelameggham N.R., Hort N. (eds) Magnesium Technology 2012 Springer, Cham, (2012) 403-408
- Gasiorowski, A. I. Mardare, N. S. Sariciftci, A. W. Hassel, J. Electroanal. Chem. 691 (2013) 77-82
- H. Meekins, A. B. Thompson, V. Gopal, B. A.Tavakoli Mehrabadi c, M. C. Elvington, P. Ganesan, T. A. Newhouse-Illige, A. W. Shepard, L. E. Scipioni, J. A. Greer, J. C. Weiss, J. W. Weidner, H. R. Colón-Mercado, Int. J. Hydrog. Energy 45 (2020) 1940-1947
- Li, H. S. Stein, K. Sliozberg, J. Liu, Y. Liu, G. Sertic, E. Scanley, A. Ludwig, J. Schroers, W. Schuhmann, A. D. Taylor, J. Mater. Chem. A 5 (2017) 67-72
- W. Hassel, M. Seo, Electrochim. Acta 44 (1999) 3769-3777





