SECM101: An Introduction to Scanning Electrochemical Microscopy
Latest updated: January 14, 2025What is Scanning Electrochemical Microscopy?
Scanning Electrochemical Microscopy (SECM) is a scanning probe technique which measures the local electrochemical activity of sample in solution. The most common form of SECM, feedback mode, measures the Faradaic current of a redox mediator which interacts with the sample, this leads to an inherent chemical selectivity.
SECM was introduced in 1989 by A. J. Bard [1], based on earlier work demonstrating that electroactive species diffusing from a biased macroelectrode could be measured by a microelectrode held within its diffusion layer [2]. Building on this work it was shown that when in close proximity to the sample the signal measured by a probe biased to interact with a redox mediator in solution was affected even without biasing the sample, and is also affected by close proximity to an insulating sample. Furthermore, the signal measured by SECM is dependent on the gap between the probe and the sample. Scanning Electrochemical Microscopy exploits this phenomenon to map both the electrochemical activity and topography of a sample.
Since its introduction and subsequent commercialization SECM has grown to become the most popular scanning probe electrochemistry technique. The flexibility afforded by the Direct Current (dc)-SECM form originally introduced has only been expanded on in recent years by the introduction of Alternating Current (ac)-SECM, and a number of constant distance SECM modes. While this article will only consider the simplest form of SECM, the feedback mode in which only the probe is biased, further information on the other SECM types can be found in our previous Learning Center article dealing with the wide array of Scanning Electrochemical Microscopy types.
How does Scanning Electrochemical Microscopy work?
In Scanning Electrochemical Microscopy an UltraMicroElectrode (UME) probe, biased at a known potential, is held near to a sample to measure the Faradaic current due to an electrochemically active species, the redox mediator, diffusing in the gap and being reduced or oxidized at the UME. The Faradaic current measured reflects the electrochemical activity of the sample. In the simplest form of SECM, feedback mode, the sample is left unbiased at Open Circuit Potential (OCP).
The UME probe of SECM is a key working principle of the technique. In SECM a diameter of 25 µm or smaller is typically used. When an UME is used hemispherical diffusion to the electrode occurs, Fig. 1, and the steady state current measured at the probe in bulk electrolyte is determined by the diffusion of the redox species to the UME probe. This means the steady-state Faradaic current measured is given by the equation below:
$i_{\mathrm{ss}}=4nFDCr$
Where iss is the steady state current, n the number of electrons transferred, F is the Faraday constant in C mol-1, D the diffusion coefficient in cm2 s-1, and C is the bulk concentration in mol cm-3 and r is the radius of the UME in cm. This equation demonstrates that the measured current is directly related to the concentration of the redox species.

Fig 1. Hemispherical diffusion to the ultramicroelectrode probe is shown.
When a biased UME probe is brought into close proximity with an insulating sample negative feedback occurs, with the diffusion of the redox mediator to the probe blocked, Fig. 2. This results in a lower concentration of redox mediator measured by the probe, and therefore a lower current compared to that in bulk solution is measured. When the SECM probe is instead over a conducting sample, the sample acts as a bipolar electrode, even if it is not biased, to recycle the redox mediator in the region under the probe, Fig. 3 and locally reach the Nernst equilibrium potential. This mediator recycling causes a local increase in the concentration of mediator detected by the probe, in turn causing an increase in the current measured.

Figure 2: Negative feedback at an SECM probe over an insulator sample is shown.

Figure 3: Positive feedback at an SECM probe over a conductor sample is shown.
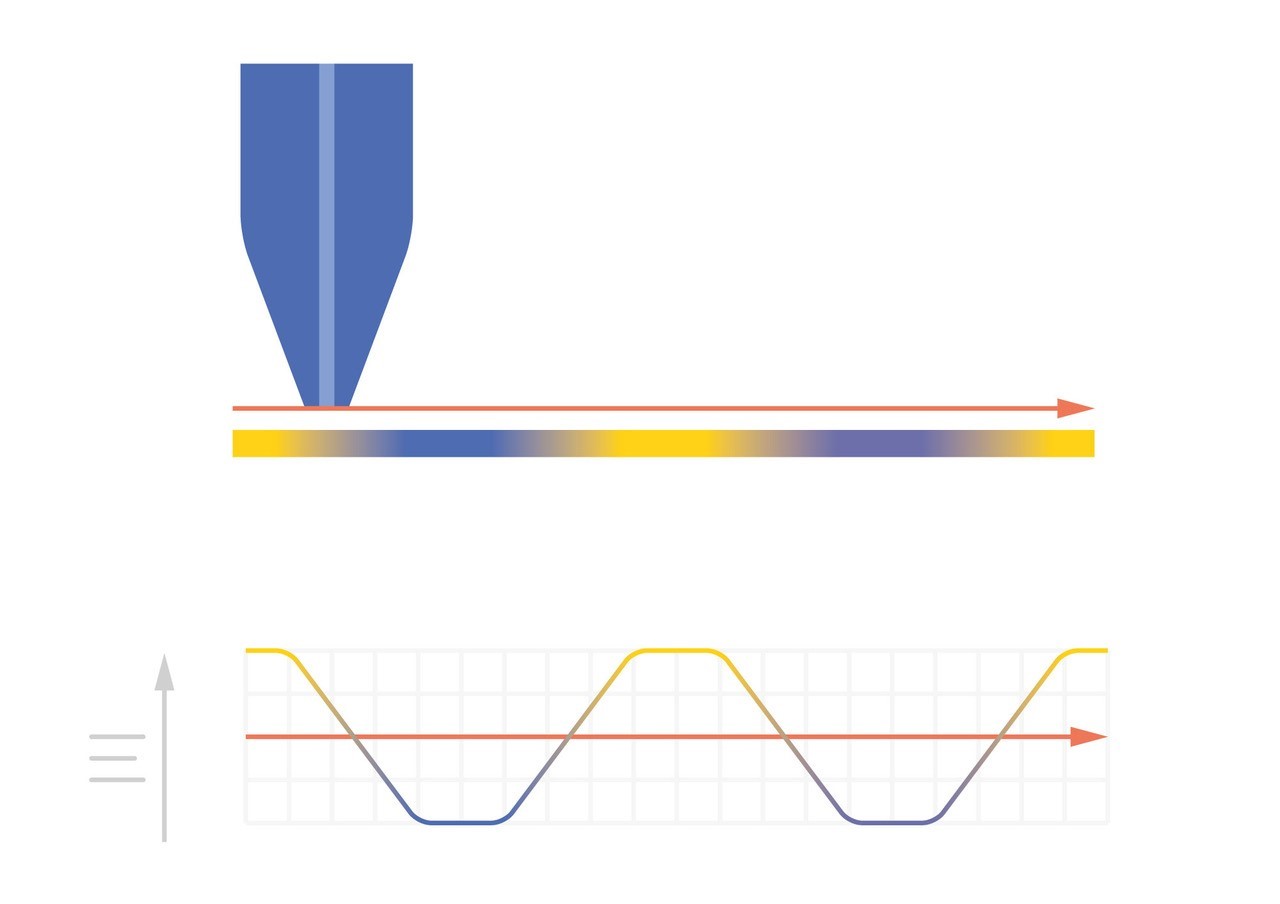
As the probe to insulating sample gap decreases the degree to which mediator diffusion is blocked increases. This results in a decrease in the absolute value of current on decreasing the probe to sample distance over an insulator. On the other hand, as the probe to conducting sample gap decreases the positive feedback from the sample increases, resulting in an increase in the absolute value of current on decreasing the probe to sample distance over a conductor. These responses are compared in Fig. 4. From this comparison it can be seen that two sample properties affect the signal measured in Scanning Electrochemical Microscopy: (1) sample activity and (2) sample topography. These different contrasts can be further demonstrated if two different sample types are considered, one a homogeneously insulating sample with topography variations, and the other a completely flat sample with heterogeneous activity. Both sample types will show variations in the SECM probe signal, but for different reasons. When the homogeneously insulating sample with topography variations is measured the probe to sample distance changes throughout the measurement. This results in a current signal absolute value which is higher over valleys, and lower over peaks due to the negative feedback at an insulator, Fig. 5. For the completely flat sample with topography variations fluctuations in the current signal are also seen. In this case the probe to sample distance remains the same throughout the measurement, as the probe moves from regions of positive feedback to those with negative feedback, and vice versa, the current measured changes, with higher absolute values of currents over the most active regions, Fig. 6. As a result, for very rough samples it can be beneficial to perform SECM measurements with a controlled probe to sample distance. A number of approaches exist to achieve constant distance SECM, though discussion of these is outside the scope of this article.

Figure 4: The difference in probe current with decreasing probe to sample distance over a conductor and insulator are compared.
 Figure 5: When SECM is used to measure a homogeneously insulating sample the variations in topography cause variations in the current measured at the probe.
Figure 5: When SECM is used to measure a homogeneously insulating sample the variations in topography cause variations in the current measured at the probe.

Figure 6: When SECM is used to measure a flat sample with varying conductivity (yellow is fully conducting, blue is fully insulating) the variations in conductivity cause variations in the current measured at the probe.
Basic Scanning Electrochemical Microscopy measurement types
There are two basic experiment types in Scanning Electrochemical Microscopy, the SECM approach curve, and the line scan, which can be used to build up an area scan of the sample.
The effect of probe to sample distance on the measured current is key to the probe approach curve experiment, in which the probe response is measured as a function of its position in z, i.e. the probe to sample distance. The approach curve serves a number of functions in SECM. Most often it is used for positioning the probe relative to the sample for a line or area scan measurement. It can also be used as a standalone experiment type. For example, approach curves have been demonstrated as an effective technique for measuring sample swelling over time [3], and under changing sample bias conditions [4]. They are also used to investigate the kinetics of reactions occurring at samples which are not easily measured by bulk electrochemistry, for example living cells and 2D materials.
The line scan experiment is an SECM measurement in which the probe is approached close to the substrate and is scanned horizontally in x or y with the probe signal plotted as a function of position. An area scan is built up through a series of x line scans performed at incremented y positions. This results in the area map correlating probe signal, usually current, to position many are familiar with for SECM measurements, an example is given in Fig. 7. The area scan is particularly useful for visualizing the local electrochemical features of a sample, and when performed over time can be used to follow the evolution of electrochemical processes.

Figure 7: SECM area scan of a spider plant leaf measured in 0.1 x 10-3 mol L-1. The 1 µm Pt probe was biased at -0.75 V to measure O2.
Though less popular than the imaging experiments, Scanning Electrochemical Microscopy is also used in measurements where the probe is approached in close proximity to the sample surface to investigate the adsorption, desorption, and dissolution of species at the sample. These measurements are performed in a stationary position using standard electrochemical measurements, and are dependent on the diffusion of species from the sample to the probe.
What are the components of a Scanning Electrochemical Microscope?
A Scanning Electrochemical Microscope is composed of a number of components which are annotated in Fig. 8. To allow the x,y,z scanning required all SECMs have an x,y,z scanning stage, capable of achieving the sub-micron step sizes required for the highest resolution measurements. Although feedback mode only requires the probe to be biased, and therefore is a single potentiostat measurement, SECM is commonly performed in other modes which also require the sample to be biased as a second working electrode. For this reason, SECMs all use a bipotentiostat. The sample is held in an electrochemical cell, on a stage, with provisions made for adjusting sample tilt. Finally, SECM requires the use of an UME probe, typically acting as the main working electrode in the measurement.

Figure 8: A Scanning Electrochemical Microscope is annotated to show all of the components.
The UME used in Scanning Electrochemical Microscopy is composed of an active electrode material surrounded by an insulating sheath, with a carefully controlled diameter. The probe tip is polished flat resulting in an active region which is a flat disc electrode. In SECM, the UME should be carefully considered due to its significant influence on the resulting measurement. The resolution of an SECM measurement ultimately depends on the diameter of the active region of the probe. Typically, the probe will be selected to be a similar scale to, or smaller than the features of interest. Although for active features which are well spaced the probe can detect features which are much smaller. The diameter also dictates how close the probe should be to the sample surface throughout a measurement. The rule of thumb is that the probe should be within 1-2 probe diameters for the best measurements, as a result probes with smaller active diameters require a smaller probe to sample distance. Also important is the ratio of the radius of the insulating sheath to that of the active material. This is known as the RG ratio and is given by the equation below:
$$RG=\frac{R}{r}$$
Where R is the radius of the insulating sheath, and r is the radius of the active area of the electrode. The RG ratio affects the sharpness of the resulting SECM image. If the RG ratio is too large diffusion of the redox mediator to the active region of the probe is blocked at larger probe to sample distances over insulating sample. While it may seem that having an RG ratio of 1 would give the best results, this is not the case. When the RG ratio is too small the diffusion of a redox mediator over an insulator is not effectively blocked. The resulting poor negative feedback response can therefore lead to a lack of distinction between the conducting and insulating regions. Typically, SECM probes have an RG ratio of 10 though this can vary. For example, the smallest probes may be made with a larger RG ratio to improve durability. A final key characteristic of the probe is the active material used. This affects the accessible electrochemical window of the probe, and the electrochemistry which can be performed at the probe. In the majority of SECM publications Pt is the probe material of choice [5].
Why use Scanning Electrochemical Microscopy?
Scanning Electrochemical Microscopy has a number of advantages. Using SECM it is possible to locally detect sample electrochemical activity. In bulk electrochemistry the measurement is an average of the entire sample, this means that local features and processes, which may have a large effect on the measurement, cannot be distinguished from other features. By using SECM it is possible to visualize these effects. An example is the use of SECM to measure the electrochemical characteristics of the grains and grain boundaries of novel materials. These features typically have different effects on the overall electrochemical characteristics of a system, which would not otherwise be easily distinguished. Furthermore, SECM is a non-contact technique which can be performed at larger probe to sample distances than other local measurement techniques, such as Atomic Force Microscopy (AFM). This is particularly advantageous when measuring fragile samples which can easily be damaged through contact, for example the solid electrolyte interface which forms on battery electrodes, or flakes of 2D materials. SECM also has no conductivity requirement for the sample, it can be used to measure anything from fully insulating to fully conducting samples, and even those with conducting regions isolated in an insulating matrix. This makes SECM highly flexible and applicable to a wide range of application areas. Furthermore, it means SECM can be used to measure samples which could be difficult to measure by bulk electrochemistry particularly because electrical contact cannot be easily made. Examples include liquid-liquid interfaces, biological materials, 2D materials, and electrode arrays for which each point can be individually examined. Finally, SECM has an inherent selectivity towards the mediator used in the measurement. This means the interaction of specific chemicals with the sample can be probed. This is of importance, for example, in the study of catalysts, antigen-sensor interactions, the release of metabolites from biological molecules, and the intercalation/deintercalation of Li + at battery electrodes.
What is Scanning Electrochemical Microscopy used for?
Figure 9: The application areas of SECM are shown.
Scanning Electrochemical Microscopy can be applied to any system measured by bulk electrochemistry for which the local electrochemical characteristics are of interest, some of the fields SECM has been used in are shown in Fig. 9. Because of its wide applicability SECM has been used in a very wide range of applications including:
- Studying corrosion processes [6]
- Analysis of coatings breakdown [7]
- Investigation of the formation of the Solid Electrolyte Interface (SEI) on battery electrodes [8]
- Screening of fuel cell catalysts [9]
- Measurement of the morphology of living cells [10]
- Investigation of ion flow through membranes [11]
- Studying the electronic properties of 2D materials [12]
These uses are covered in more detail in a series of Learning Center articles focusing on the different research questions which can be answered by scanning probe electrochemistry. The latest article in this series focuses on the use of scanning probe electrochemistry in fuel cell research.
Further Information
BioLogic® has a number of resources available dedicated to taking your understanding of Scanning Electrochemical Microscopy further. To learn more about the use of SECM in different application areas please consult the scanning probe workstations application notes. For information on achieving the best images in SECM please see our two-part tutorial: How to get clear images in SECM.
Glossary
- Faradaic current: The current related to an oxidation or reduction reaction.
- Feedback mode Scanning Electrochemical Microscopy: The simplest form of SECM in which the probe is biased to measure the interaction of a redox mediator in solution, with an unbiased sample.
- Positive feedback: The increase in redox mediator concentration, and associated increase in probe current, which occurs over a conducting sample due to mediator recycling.
- Negative feedback: The decrease in redox mediator concentration, and associated decrease in probe current, which occurs over an insulating sample due to blocked mediator diffusion.
- Redox mediator: An electrochemically active compound which transfers electrons in SECM, allowing measurement of Faradaic current.
- RG ratio: The ratio of the radius of the insulating sheath to the radius of the active probe region.
- Scanning probe electrochemistry: Any of the family of scanning probe techniques which measures the local electrochemistry of a sample. This includes SECM, SKP, LEIS, SDC, and SVET.
References
- J. Bard, F. R. F. Fan, J. Kwak, O. Lev, Anal. Chem. 61 (1989) 132-138
- C. Engstrom, M. Weber, D. J. Wunder, R. Burgess, S. Winquist, Anal. Chem. 58 (1986) 844-848
- Elkebir, S. Mallarino, D. Trinh, S. Touzain, Electrochim. Acta 337 (2020) 135766
- Fic, A. Płatek, J. Piwek, J. Menzel, A. Ślesiński, P. Bujewska, P. Galek, E. Frąckowiak, Energy Storage Mater. 22 (2019) 1-14
- Polcari, P. Dauphin-Ducharme, J. Mauzeroll, Chem. Rev. 116 (2016) 13234−13278
- Kong, C. Dong, Z. Zheng, F. Mao, A. Xu, X. Ni, C. Man, J. Yao, K. Xiao, X. Li, Appl. Surf. Sci. 440 (2018) 245–257
- Morimoto, A. Fujimoto, H. Katoh, H. Kumazawa. AIAA Scitech 2019 Forum, 7-11 January 2019, San Diego, California
- Liu, Q. Yu, S. Liu, K. Qian, S. Wang, W. Sun, X.-Q. Yang, F. Kang, B. Li, J. Phys. Chem. C 123 (2019) 12797-12806
- Black, J. Cooper, P. McGinn, Meas. Sci. Technol. 16 (2005) 174
- Razzaghi, J. Seguin, A. Amar, S. Griveau, F. Bedioui, Electrochim. Acta 157 (2015) 95–100
- J. Jackson, J. M. Sanderson, R. Kataky, Sens. Actuators B Chem. 130 (2008) 630–637
- Henrotte, T. Bottein, H. Casademont, K. Jaouen, T. Bourgeteau, S. Campidelli, V. Derycke, B. Jousselme, R. Cornut, ChemPhysChem 18 (2017) 2777 – 2781

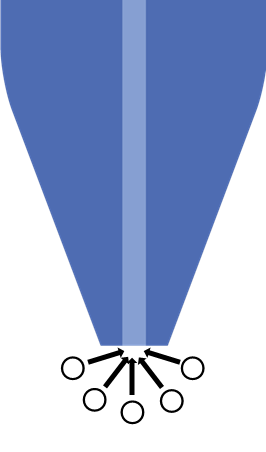
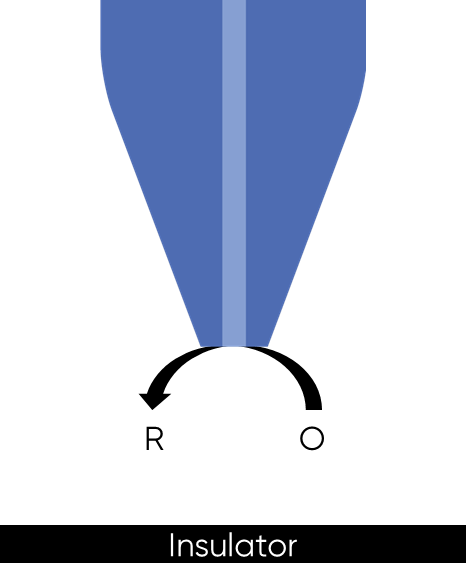

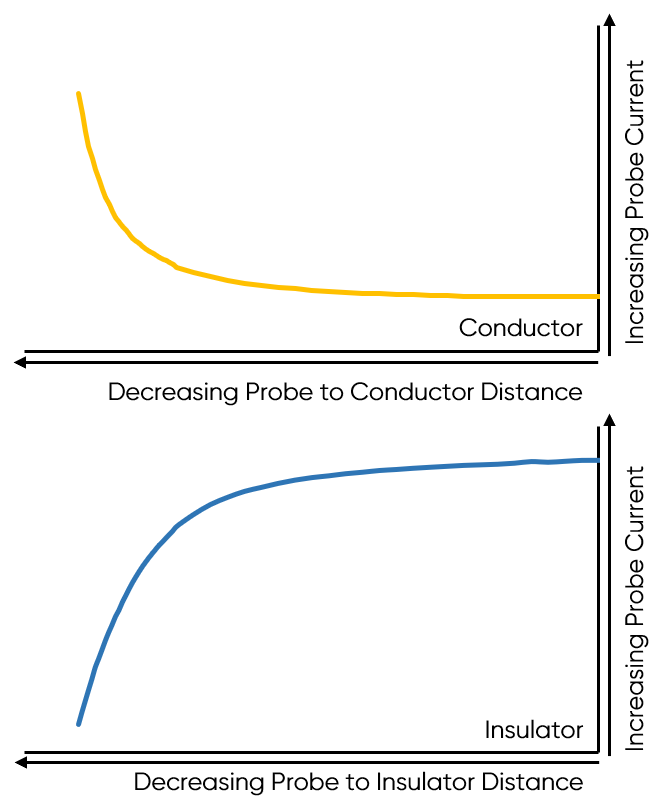
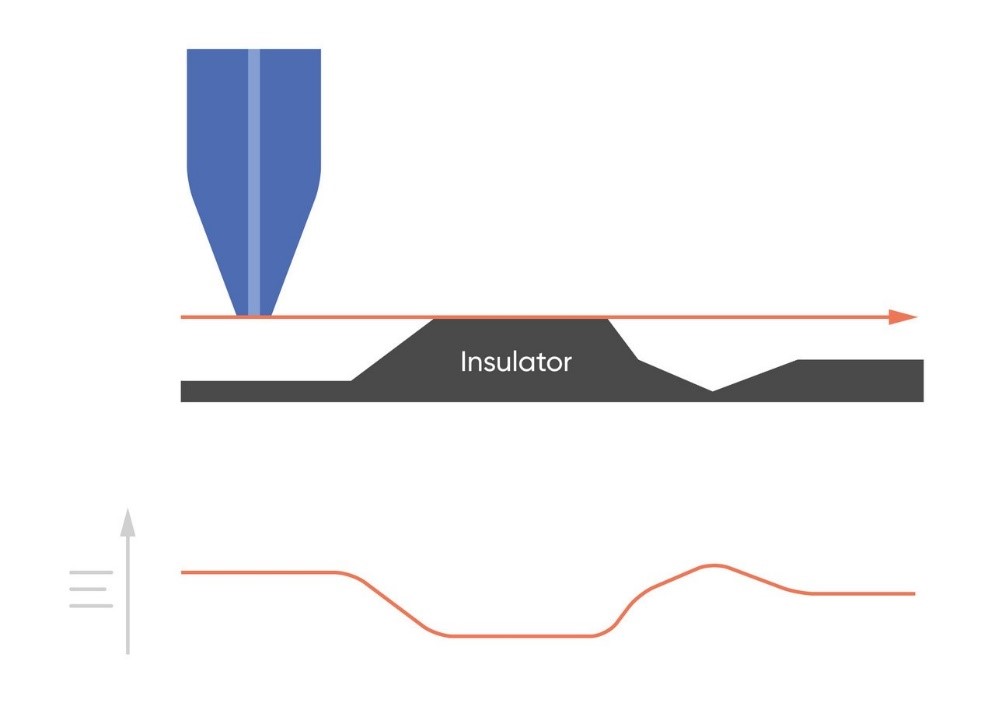 Figure 5: When SECM is used to measure a homogeneously insulating sample the variations in topography cause variations in the current measured at the probe.
Figure 5: When SECM is used to measure a homogeneously insulating sample the variations in topography cause variations in the current measured at the probe.