The electrical conductivity of solid electrolytes
Latest updated: November 22, 2024The efficiency and performance of energy storage devices depend on the electrical properties of the materials used for the anode, cathode and for the electrolyte. The most commonly investigated electrical property is electrical conductivity, which quantifies the ability of the material to transport charged species, (called charge carriers), in the presence of an electrical field. Electrical conductivity, an intrinsic and intensive physical property of a material, depends on the temperature and sometimes on the humidity and the direction/orientation at which the measurement is performed. So, for reliable conductivity measurements, these parameters must be controlled.
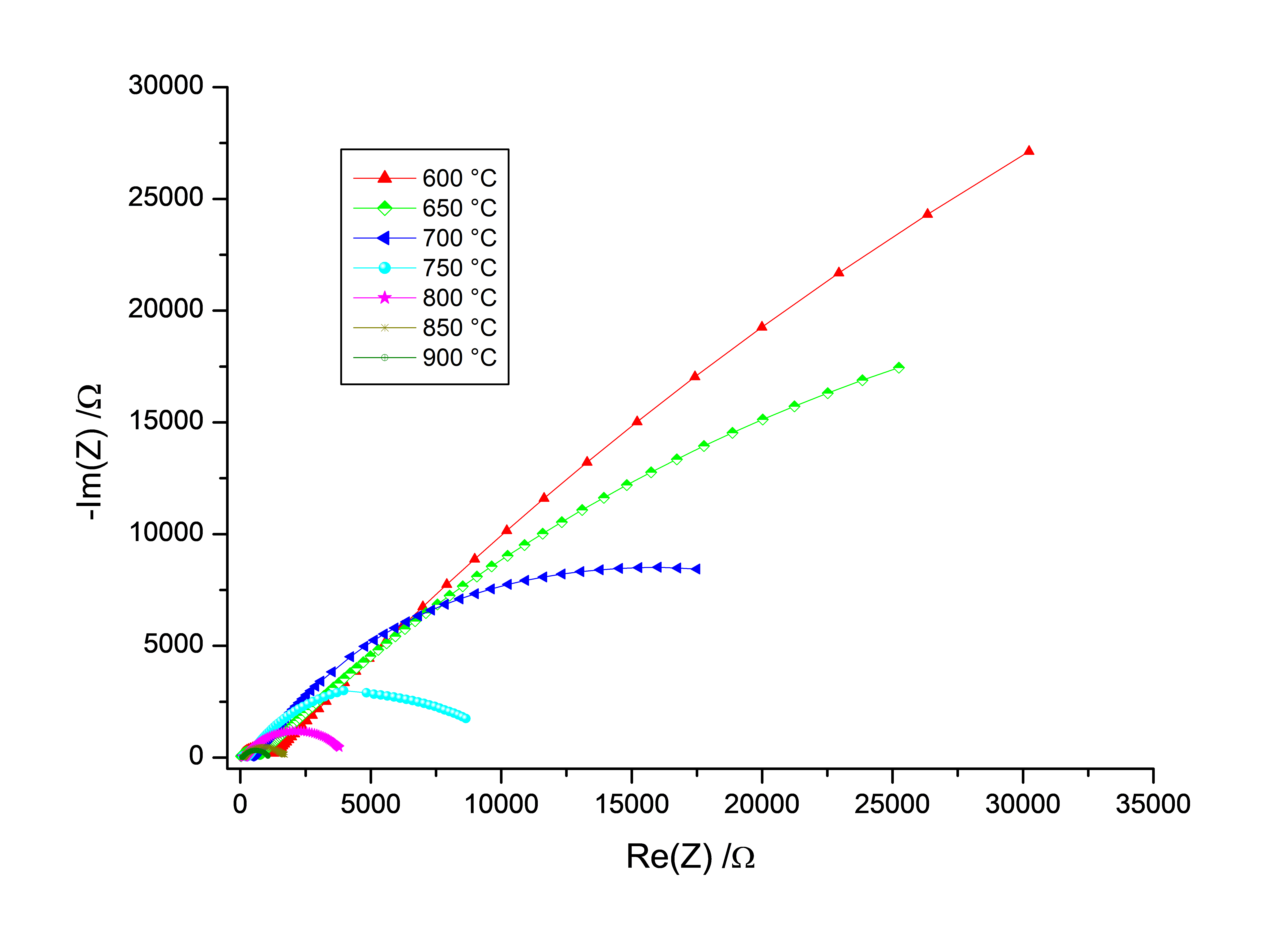
AC conductivity is mainly determined by processing Impedance data obtained by impedance spectroscopy measurement. Thus, an impedance analyzer with a large frequency range is needed to investigate the conduction mechanisms of different kinds of materials (polymers, ceramics, composites, etc). For the purpose of illustration, the through-plane AC conductivity of Yttria Stabilized Zirconia electrolyte, used as a solid electrolyte material for SOFC, was investigated by Impedance spectroscopy at high temperatures (from 600 °C to 900 °C) using an integrated solution, consisting of an impedance analyzer, a high temperature furnace and a high temperature sample holder.
The Bulk and grain boundary conductivities, the activation energy for the bulk and the grain boundary conduction were extracted from the AC impedance data at each temperature.
Read More …
Related products
The following products are relevant to this article.
Related accessories
The following accessories are relevant to this article.




