What are All-Solid-State Batteries
Latest updated: May 22, 2025Introduction
All-solid-state batteries (ASSBs) have emerged as a promising solution to address the limitations of traditional lithium-ion batteries (LIBs). These batteries offer the potential to revolutionize industries ranging from electric vehicles to renewable energy systems. By replacing the liquid electrolyte found in LIBs with solid materials, ASSBs aim to enhance safety, increase energy density, and extend the overall lifespan of energy storage systems. In this article, we’ll introduce all-solid-state batteries, similarities and differences to LIBs, ongoing research challenges, and instrumentation requirements.
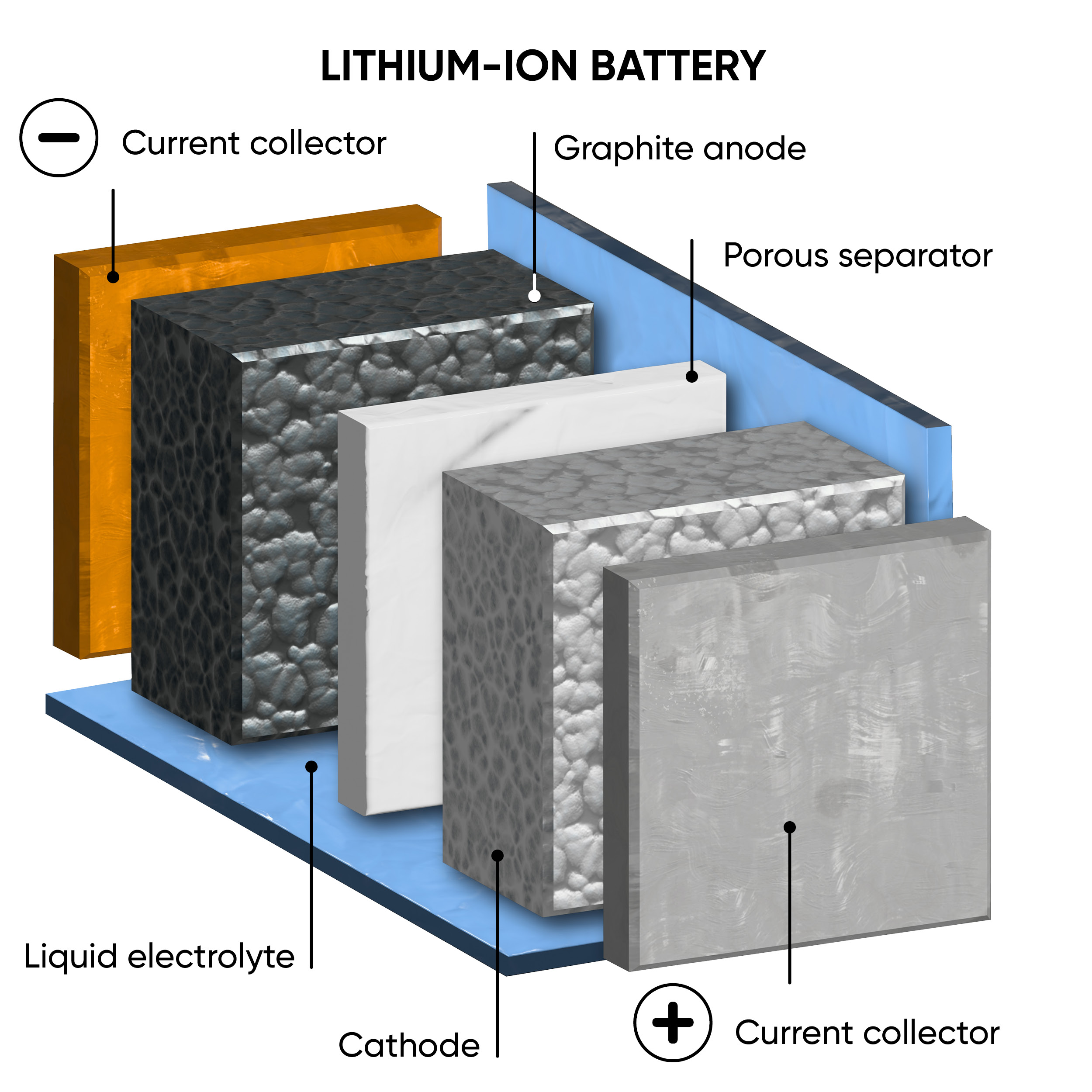
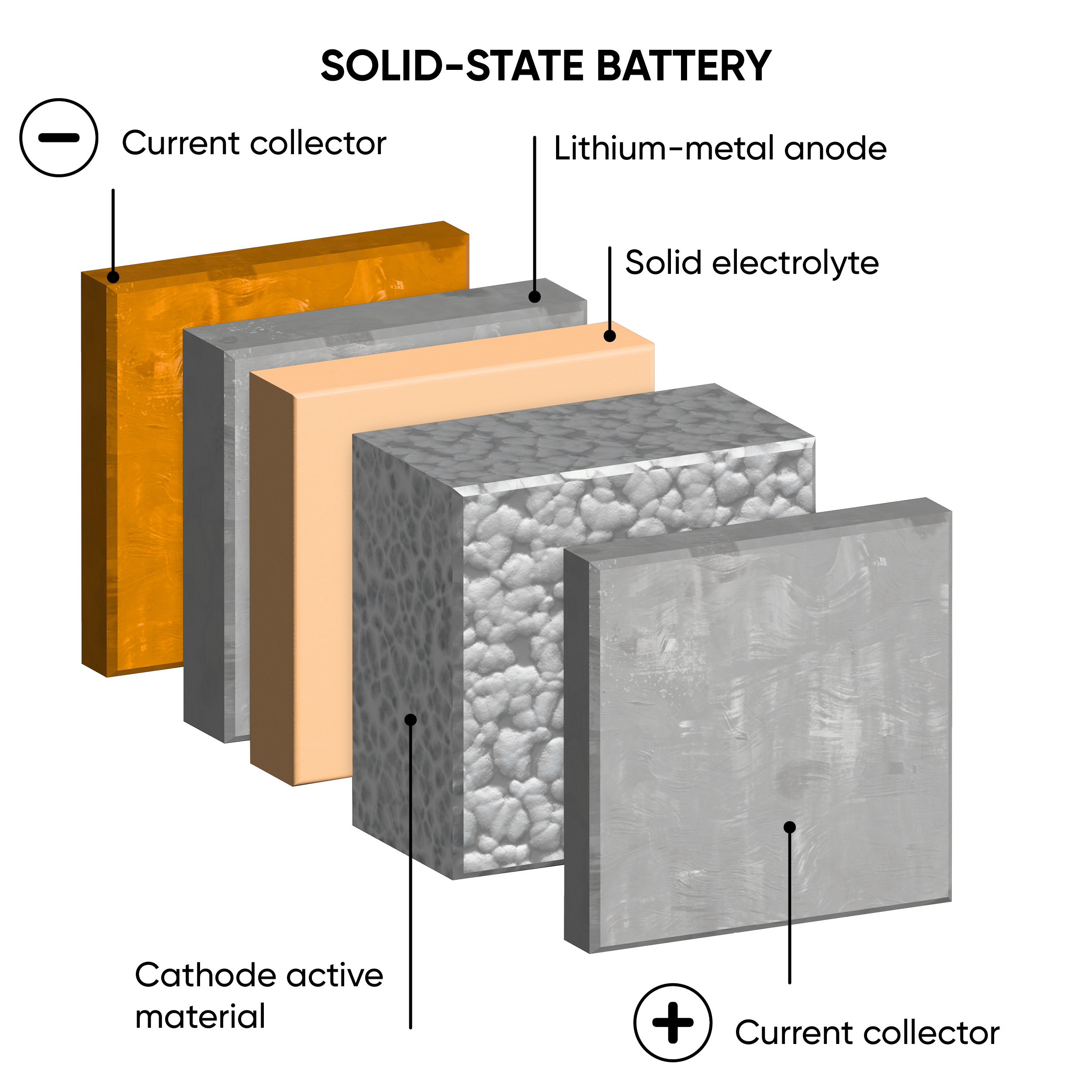
The main difference between ASSBs and LIBs is the state of their electrolytes. Traditional LIBs have a liquid or gel electrolyte, whereas ASSBs employ solid electrolytes. Figure 1 illustrates the structural distinctions between ASSBs and LIBs. The positive and negative electrodes act as either anode or cathode depending on whether the device is charging or discharging. A range of solid electrolytes are currently being explored and include ceramics, polymers, resins and glass composites. [1] There are some safety concerns posed by LIBs due to their flammable nature that may be improved by ASSBs by reducing the risk of leakage, thermal runaway, and dendrite formation.
In addition to improving safety, all-solid-state batteries also offer higher energy densities compared to traditional LIBs. This means that ASSBs can store more energy in the same amount of space, making them especially attractive for applications that require compact energy storage solutions like electric vehicles and portable electronics. Having unique transport, thermal and mechanical characteristics, ASSBs can also potentially address the major issues encountered with LIBs and overcome fast charging limitations. [2]


Figure 1: A schematic comparison between the structure of a traditional lithium-ion battery (left) and an all-solid-state battery (right), during discharge.
Research Endeavors and Obstacles
The transition from liquid to solid electrolytes introduces its own set of challenges. Some of these challenges include:
- Reduced conductivity of solid electrolytes at room temperature.
- Sluggish kinetics at interfaces.
- Fabricating and maintaining defect-free interfaces
Reduced conductivity of solid electrolytes at room temperature
Solid-state batteries exhibit lower ionic conductivity compared to traditional liquid electrolyte batteries due to the inherent nature of solid electrolytes. Ions are not as free to move around in solids, or even polymers, as they are in liquids because ions must move through lattices and grain boundaries. Conductivities of Li+ solid electrolytes tend to be 2-4 orders of magnitude lower than liquid electrolytes. [3] This decreased mobility is related to a higher activation energy barrier and is temperature dependent. The bulk conductivity follows the Arrhenius law relating the DC ionic conductivity, σ, and temperature, T through equation 1:
$$ \sigma = \sigma_0 \exp\left(-\frac{E_\text a}{K_\text b T}\right)$$
Where:
- $\sigma$ is the DC ionic conductivity (S·m-1)
- $\sigma_0$ is the pre-exponential factor (S·m-1)
- $E_\text a$ is the activation energy (J)
- $K_\text b$ the Boltzmann constant (8.61 x 10-5 eV·K-1)
- $T$ is the absolute temperature (K)
At higher temperatures, ions have more thermal energy, which helps them overcome the activation energy barriers and move more freely through the solid electrolyte lattice. As a result, the ionic conductivity of solid electrolytes increases with higher temperatures.
Sluggish kinetics at interfaces
Higher impedance is observed at the interface in ASSBs because of the complex nature of solid-solid interfaces and the inherent challenges associated with ion transport through solid electrolytes. This is due to a combination of factors, both electrochemical and physical, including reduced ion transport, charge transfer kinetics, and interfacial layer formation. [3]
Fabricating and maintaining defect-free interfaces
Another challenge is in the fabrication process because uniform and defect-free interfaces between electrode and electrolyte materials are important for maintaining battery performance. ASSBs can also experience defects due to expansion and contraction as they undergo charge and discharge cycles. This phenomenon, known as volume or dimensional change, is primarily caused by the insertion and extraction of ions within the solid electrode materials during electrochemical reactions. These volume changes can cause mechanical stress, capacity fade, dendrite formation and interface instability, which can impact the overall cycle life of the battery by causing irreversible damage to the materials and even catastrophic failure of the solid electrolyte-electrode interface.
Many promising solid electrolyte materials have been identified, but still need to be optimized for performance, stability and ease of manufacturing.

Figure 2: Range of electrochemical instruments used in the research and testing of ASSBs.
Developing and testing ASSB technology
Potentiostats, impedance analyzers and battery cyclers are fundamental for testing both LIBs and ASSBs and are used through the full battery value chain: from materials and components research to full cell testing and validation. Figure 2 provides an example of the range of electrochemical instruments needed at each stage of ASSB development and testing. During development and testing phases, ASSBs require specific testing environments to determine optimal operational conditions and often need to be incorporated into high-end measurement chains for in situ/operando measurement techniques, including the following.
- X-ray Diffraction (XRD): Helps researchers analyze the crystal structures of materials, providing insights into their stability and behavior during charging and discharging cycles.
- Scanning Electron Microscopy (SEM): Enables researchers to examine the microstructure of battery components, aiding in identifying defects or irregularities that could affect performance.
- Scanning Electrochemical Microscopy (SECM): Offers researchers the opportunity to study the local electrochemistry of a sample, in particular the study of the local differences in conductivity of grain and grain boundaries.
- Thermal Analysis Techniques: These techniques help researchers understand thermal behavior and stability of battery materials.
By providing precise control over the electrochemical processes, these systems enable researchers to better study battery component properties and evaluate performance under a variety of conditions.
Conclusion
The development of all-solid-state batteries represents a significant step forward in energy storage technology. Their potential to enhance safety, increase energy density, and enable new applications holds promise for a more sustainable and efficient energy future. While challenges remain in terms of material development, manufacturing, and performance optimization, ongoing research efforts and advanced instrumentation are steadily pushing the boundaries of this technology. As these hurdles are overcome, all-solid-state batteries could reshape industries and play a pivotal role in the global transition to cleaner and more efficient energy systems.
References:
[1] Lim HD, Park JH, Shin HJ, Jeong J, Kim JT, Nam KW, et al., ‘A review of challenges and issues concerning interfaces for all-solid-state batteries’, Energy Storage Mater, vol. 25, (2020) 224–250
[2] Vishnugopi BS, Kazyak E, Lewis JA, Nanda J, McDowell MT, Dasgupta NP, et al, ‘Challenges and Opportunities for Fast Charging of Solid-State Lithium Metal Batteries’, ACS Energy Letters, vol. 6, no. 10. American Chemical Society, (2021) 3734–3749
[3] Lou S, Zhang F, Fu C, Chen M, Ma Y, Yin G, et al, ‘Interface Issues and Challenges in All-Solid-State Batteries: Lithium, Sodium, and Beyond’, Advanced Materials, Wiley-VCH Verlag, vol. 33, no. 6. (2021)