What is a potentiostat and its use in Science & Industry (Electrochemistry Basics Series)
Latest updated: June 24, 2025Definition
A potentiostat, also known as a galvanostat or electrochemical workstation, is an electronic instrument used to control and measure the voltage and/or current in an electrochemical cell. Potentiostats are essential tools in electrochemistry and play a critical role in both scientific research and industrial applications.
What Does a Potentiostat Do?
A potentiostat is an essential instrument in electrochemistry that enables researchers to precisely investigate and manipulate electrochemical reactions, making it invaluable across a wide range of scientific and industrial applications.
Key Applications:
- Electrochemical analysis
- Battery research and testing
- Corrosion studies
- Chemical synthesis & Electrocatalysis
- Materials science
- Bioelectrochemistry

Note:
Potentiostats are also widely used in Electrochemical Impedance Spectroscopy (EIS), a powerful technique for measuring the resistance and capacitance of electrochemical systems.
Definition of a potentiostat in video
How Does a Potentiostat Work?
A potentiostat controls the potential between a working and a reference electrode while measuring the resulting current flowing to a counter electrode. Depending on whether it is controlling voltage or current, the device operates in either potentiostatic or galvanostatic mode.
Potentiostatic Mode
In this mode, the potentiostat controls the voltage applied to the electrochemical cell and measures the current (see Figure 1 below). It is especially common in corrosion and analytical electrochemistry.
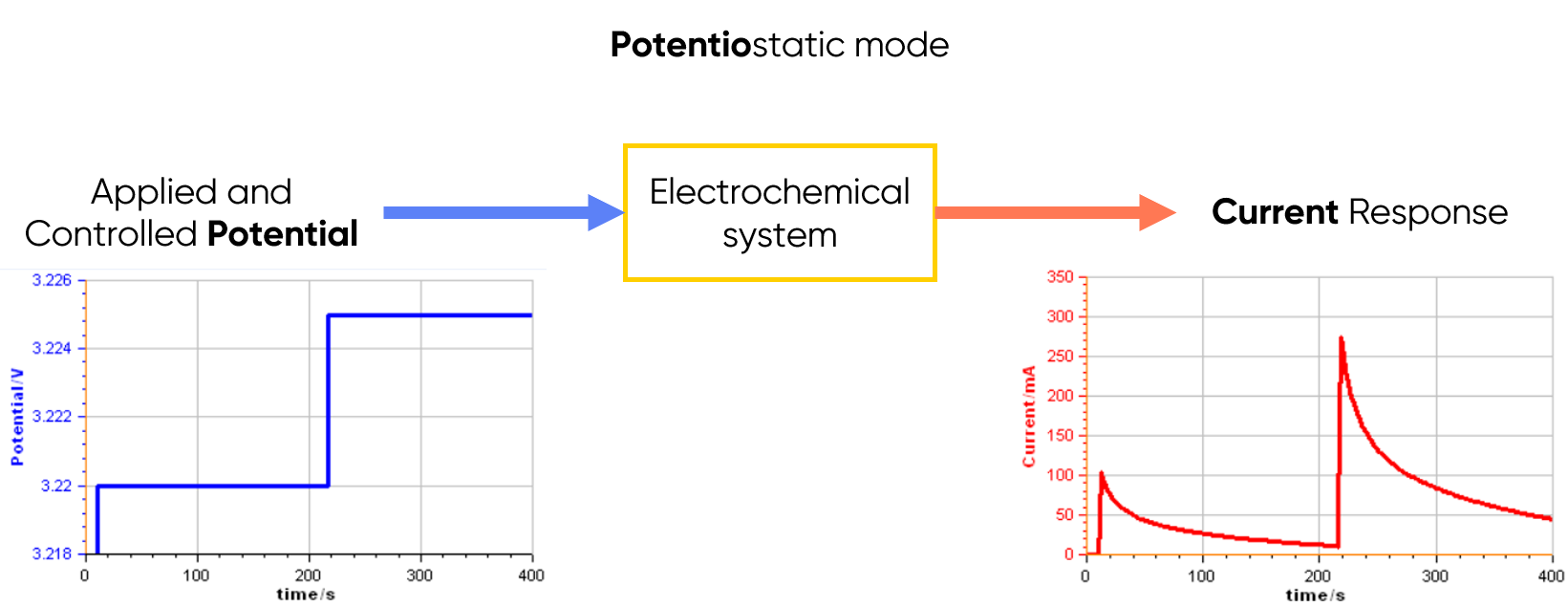
Figure 1: Principle of the potentiostatic mode.
Use case: Applying a “voltage ramp” (e.g. Cyclic Voltammetry (CV))
Galvanostatic Mode
In galvanostatic mode, the device controls the current and measures the voltage response. This is the primary mode used in battery research and materials testing.

Figure 2: Principle of the galvanostatic mode.
Use case: Charging/discharging batteries during cycling experiments
Modern potentiostats often switch between modes to meet complex testing needs, especially in battery cycling protocols. All BioLogic potentiostats are equipped with fast CCCV shift, making data more reliable.
Other Control Modes
- Open Circuit Voltage (OCV): Measures cell voltage with no current applied. This control mode is commonly used for equilibration of the electrochemical cell.
- Electrochemical Impedance Spectroscopy (EIS): Applies a sinusoidal signal (current or voltage) and analyzes the system’s response. This control mode is widely used in electrochemical and corrosion systems because it provides detailed information about reaction kinetics, corrosion rates, and mass transfer parameters, amongst others.
- Zero Resistance Ammeter (ZRA): Applies 0 V between two electrodes and measures the current flow. This control mode allows the determination of electrochemical current noise. More information about the electrochemical noise measurements can be found in Application Note #39-1.#39-2, #39-3
How is the cell connected?
The Three-Electrode Setup
A standard electrochemical experiment uses three electrodes:
- Working Electrode (WE): Site of the electrochemical reaction
- Reference Electrode (RE): Maintains a stable, known potential
- Counter Electrode (CE): Completes the circuit by balancing the current
To guarantee that currents flows as a result of potential variations at the working electrode interface, the potential of the reference electrode must remain stable and correspond to its theoretical value. It must be properly maintained. (see Checking and Validating reference electrodes).
Figure 3: Example of a three-electrode setup.
What is the Potentiostat made of?
To manage the current and voltage, the potentiostat is composed of two main electronic components:
Control Amplifier (CA): A core electronic component that regulates the potential between the WE and RE, keeping it as close as possible to the voltage of the input source $E_\text i$. The potentiostat circuit diagram in Figure 4 shows the position of the control amplifier in a simplified design of a modern potentiostat [1].
Feedback Loop: The voltage from the reference electrode is fed back to the control amplifier to maintain the setpoint. This creates a loop called the “negative feedback loop”, which allows the control amplifier to adapt its output and maintain a potential difference corresponding to $E_\text i$. For more information, see the BioLogic Application Note #04: “The mystery of potentiostat stability explained”.
Figure 4: Circuit diagram of basic potentiostat design [1].
A Brief History of the Potentiostat
- 1903: F.G. Cottrell introduces early potentiostatic concepts while studying mass transfer. His setup used a battery and galvanometer but lacked control over electrode potential.
- 1942: A. Hickling, at the University of Leicester, develops the first modern three-electrode potentiostat [3] using negative feedback to control electrode potential precisely.
- 1956: Electrochemist Prazak coins the term potentiostat to describe Hickling’s instrument.
- Late 1950s–1960s: Hans Wenking improves potentiostat electronics, solving key design challenges and influencing modern workstation architecture [4].
- 1971: Control amplifiers are introduced [5], enabling more precise and stable implementation of negative feedback.
- 1970s onward: Electrochemical impedance spectroscopy (EIS) becomes a common technique, driving advances in potentiostat functionality and performance.
- 1991: BioLogic launches the MacPile, the first multichannel computer-controlled potentiostat, developed by Yves Chabre and Christian Mouget (see Figure 5).
Figure 5: The world’s first multichannel computer-controlled potentiostat –The Mac Pile, launched in 1991.
Today’s potentiostats offer advanced software integration, fast switching between modes, and high measurement accuracy.
Potentiostat Applications in Research & Industry
Energy Storage
The advances made in battery research over the past decade have been remarkable. In the early 2000’s, lithium-ion technology experienced exponential growth, driven by the telecommunications boom and widespread adoption of mobile devices. Today, lithium-ion batteries power everything from smartphones to electric vehicles.
Potentiostats (and Battery Cyclers) are essential tools in battery research and development. They allow for precise analysis of a cell’s electrochemical behavior, including:
- Charge/discharge profiling under controlled conditions
- Capacity retention and aging analysis
- Electrochemical Impedance Spectroscopy (EIS) to assess internal resistance and interface stability
These measurements help researchers optimize battery chemistries, electrode materials, and cell design. Every component—from electrodes and separators to electrolytes and full cells—must be thoroughly characterized. BioLogic offers an extensive range of instruments to support every stage of battery development.
The role of Battery Cyclers
Battery Cyclers complement potentiostats by focusing on two main tasks: ensuring battery quality and quantifying performance (e.g., capacity and lifetime). Their primary functions include:
- Measuring charge and discharge capacity
- Evaluating cycle life and degradation behavior including State of Charge (SoC), State of Health (SoH)
- Quantifying efficiency and energy losses during repeated use
By running controlled cycling protocols, battery cyclers help determine how a cell behaves under different current loads, temperatures, and usage patterns. This data is vital for assessing product quality, verifying manufacturer specifications, and supporting research into improved battery chemistries and cell designs.
As batteries age, their internal resistance tends to increase, which affects performance and lifespan. Techniques such as Electrochemical Impedance Spectroscopy (EIS), often performed during or between cycles, allow for a more detailed understanding of aging processes and help inform strategies for battery reuse, upcycling, or end-of-life management—collectively known as a battery’s “second life”
Battery Market Insight: The global battery market is projected to reach $279.7 billion by 2027, underscoring the increasing role of potentiostats in energy storage innovation.
Corrosion Science
In corrosion research, potentiostats allow precise control and monitoring of electrochemical conditions to:
- Quantification of corrosion rates using techniques like Linear Polarization Resistance (LPR)
- Assessment of coatings and corrosion inhibitors
- Investigation of localized corrosion using techniques such as LEIS or SECM
This data is essential for designing durable materials for infrastructure, marine, aerospace, and energy sectors.
Investigating local corrosion effects
Scanning probe workstations, such as BioLogic’s M470, provide high-resolution insights into corrosion processes at the microscale—complementing the macroscale view offered by standard potentiostats. Techniques like Localized Electrochemical Impedance Spectroscopy (LEIS) allow researchers to map impedance distribution across a surface, identifying weak points and assessing protective layer integrity (see: “Coatings, Corrosion and Scanning Probe Electrochemistry”).
Corrosion Insight: The global cost of corrosion is estimated at $2.5 trillion [7], with potential savings of $375–875 billion annually through effective corrosion control—equivalent to 3.4% of global GDP (2013).
Electrocatalysis and Fuel Cells
Potentiostats play a key role in electrocatalysis research, particularly in the development of fuel cells, electrolyzers, and CO₂ reduction systems. These technologies are central to clean energy conversion and storage. Applications include:
- Evaluating catalyst activity and stability for reactions such as the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER)
- Tafel analysis and Cyclic Voltammetry (CV) to assess reaction kinetics
- Electrochemical Surface Area (ECSA) estimation for benchmarking catalyst performance
- Long-term stability testing of fuel cell components under load cycling and environmental conditions
Potentiostats enable researchers to optimize catalyst formulations, membrane materials, and electrode architectures. When combined with techniques like rotating disk electrode (RDE) testing or EIS, they provide detailed insights into reaction mechanisms and system inefficiencies.
Fuel Cell Insight: As governments and industries aim for decarbonization, fuel cells are gaining traction across transportation, backup power, and industrial sectors—driving demand for advanced electrochemical testing tools.
Fundamental Electrochemistry
In both academic and industrial labs, potentiostats are essential for probing redox reactions and reaction mechanisms. Core techniques include:
- Cyclic Voltammetry (CV) to investigate electron transfer pathways
- Chronoamperometry and Chronopotentiometry for time-dependent studies
- Quantifying electrocatalytic efficiency in sensors and environmental monitoring applications
These foundational measurements support innovations in energy, biosensing, materials science, and more.
How to Choose a Potentiostat
Choosing the right potentiostat depends on your research or industrial needs:
- Voltage and Current Ranges: Match to your system requirements
- Electrochemical Impedance Spectroscopy Capability: Frequency range and accuracy matter for impedance studies
- Number of Channels: Single vs. multichannel systems for parallel experiments and group work
- Scan Rate & Sampling Speed: For fast reactions or transient analysis
- Integration: Compatibility with climate chambers, spectrometers, etc.
| Feature | Consideration |
|---|---|
| Max Current | Required for high-load applications |
| Min Current | Critical for low-current measurements |
| Compliance Voltage | Determines range of usable working voltage |
| Number of Channels | Need for multielectrode setups, group work, bi-pot mode (e.g. RRDE) |
| Software | Important for automation and data analysis Ergonomics, Data Display, Data Analysis capability |
Summary
Potentiostats are versatile instruments at the heart of modern electrochemical research and industrial quality control. Whether you’re studying corrosion, developing next-gen batteries, or creating biosensors, potentiostats provide the precision and control needed to push the boundaries of science and technology.
Discover our advanced BioLogic potentiostat in video
Discover our advanced BioLogic potentiostat range in detail
BioLogic Potentiostat Discovery Page
References
- Application Note #04 “The mystery of potentiostat stability explained”.
- A.Hickling, Studies in electrode polarisation, Part IV, (1942).
- A.Hickling, Electrochimica Acta, Vol 5, (1961) 161-168.
- R.Dölling, Materials and Corrosion, 49, (1998) 535-538.
- B. Petrescu, Système électroanalytique flexible contrôlé par ordinateur, Thèse de l’Institut National Polytechnique de Grenoble et de l’Université “Politehnica” de Bucarest, 2002.
- S. Madhu, A. J. Anthuuvan, S. Ramasamy, P. Manickam, S. Bhansali, P. Nagamony, V. Chinnuswamy, ACS Applied Electronic Materials, 2, (2020) 499-509.
- International measures of prevention, application, and economics of corrosion technologies study, Nace International report
- H-R. Erfanian-Nazif’Toosi, H. F. Lopez: Journal of Materials and Applications, 9(1), (2020) 1-8.
- T. Liu, Y. Wang, S. Pan, Q. Zhao, C. Zhang, S. Gao, Z. Guo, N. Guo, W. Sand, X. Chang, L. Dong, Y. Yin, Corrosion Science, 149, (2019) 153-163.
- Global Battery Market Report 2020-2027, ResearchAndMarkets.Com
Glossary
| Term | Definition |
| Battery cycling | Testing process technique of batteries based on repeated and successive charge and discharge phases. |
| Control amplifier | Main active electronic device & part of the analog control loop of a potentiostat, delivering power to an electrochemical cell . |
| Current | Physical quantity describing the flow of charged particles (electrons, ions) in a conductor (SI unit: A) |
| Counter electrode | Auxiliary electrode allowing current to flow through the cell. |
| Electrode potential (Voltage) | The quantity which describes the potential difference between both sides of the electrode interface (SI unit: V). |
| Internal resistance | Generic term that does not designate a specific resistance in the battery. It is a loose characteristic of the battery. |
| Negative feedback | The loop operated by the control amplifier. |
| Potentiostat / Galvanostat | Electronic device capable of applying a voltage and measuring the current response (or vice versa) of an electrochemical interface. |
| Reference electrode | Electrode used to measure the potential difference of an electrochemical interface. Its own potential is stable because it is not traversed by a current. |
| Working electrode | An electrode on which the reaction of interest occurs. |

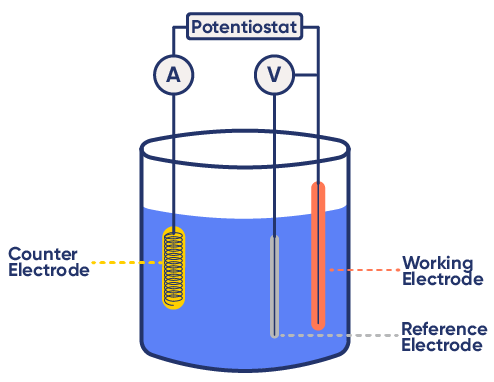
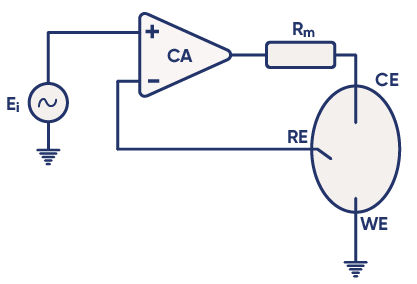
 Figure 5: The world’s first multichannel computer-controlled potentiostat –The Mac Pile, launched in 1991.
Figure 5: The world’s first multichannel computer-controlled potentiostat –The Mac Pile, launched in 1991.