What is CO2 electrolysis?
Latest updated: April 11, 2025Introduction
Carbon dioxide or CO2 is the single most abundant greenhouse gas and CO2 levels are higher than they have been for millions of years (NOAA). Reducing CO2 emissions is a multifaceted and complicated issue, but one promising solution is CO2 electrolysis—a process that transforms CO2 into valuable chemicals and fuels using electricity1,2. This technology not only offers a way to mitigate CO2 emissions but also presents an opportunity to integrate renewable energy sources into the production of essential chemicals.
By understanding CO2 electrolysis, we can better appreciate its role in a sustainable future. In this article, we will explain the basic principles of CO2 electrolysis, its products and potential benefits, its research and development, and the challenges and prospects of this promising technology3.
CO2 Electrolysis
CO2 electrolysis is the electrochemical reduction of CO2 into various products. The specific product depends on factors including the type of cell and electrodes, the catalyst used and the reaction conditions (including voltage, temperature, and pressure).
These reactions are performed in an electrolyzer, which is a cell designed for CO2 reduction reactions (CO2RR). There are many different designs including H-type cells, gas diffusion electrodes (GDE) cells, membrane electrode assembly (MEA) cells and solid-state electrolyte cells4–6.
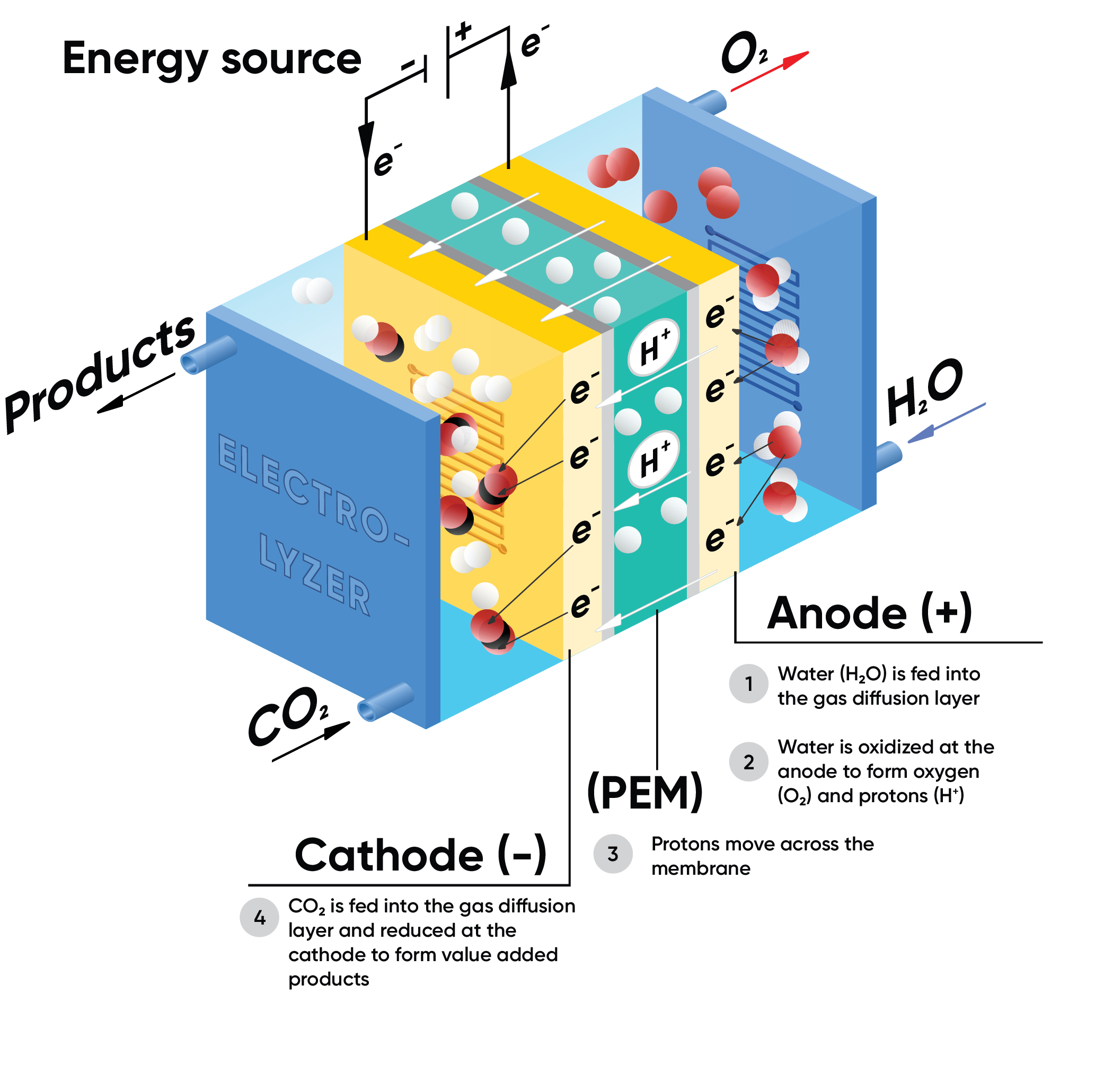
Figure 1: Example of a CO2 electrolyzer.
Similar to PEM electrolyzers used for hydrolysis, a typical electrolyzer for CO2RR consists of the following:
Cathode: Where CO2 reduction occurs, made from electrodes like copper, silver, or specialized catalysts that enhance the selectivity and efficiency of the reaction7.
CO2 molecules gain electrons and react with $\text H ^+$ to form products such as carbon monoxide (CO), methane (CH4), ethylene (C2H4), or alcohols like ethanol (C2H5OH) and water.
The corresponding electrochemical reactions, along with the standard electrode potentials vs SHE (Standard Hydrogen Electrode), are shown below8:
| Half-cell reaction | Eo/(V/SHE, pH=7) |
|---|---|
| $2\text{H}^+ + 2\text{e}^- \rightarrow \text{H}_2$ | -0.42 |
| $\text{CO}_2 + 2\text{H}^+ + 2\text{e}^-\rightarrow \text{CO} + \text{H}_2\text{O}$ | -0.53 |
| $\text{CO}_2 + 2\text{H}^+ + 2\text{e}^-\rightarrow \text{HCOOH}$ | -0.61 |
| $\text{CO}_2 + 6\text{H}^+ + 6\text{e}^-\rightarrow \text{CH}_3\text{OH}+ \text{H}_2\text{O}$ | -0.38 |
| $\text{CO}_2 + 8\text{H}^+ + 8\text{e}^-\rightarrow \text{CH}_4+ 2\text{H}_2\text{O}$ | -0.24 |
| $2\text{CO}_2 + 12\text{H}^+ + 12\text{e}^-\rightarrow \text{C}_2\text{H}_4+ 4\text{H}_2\text{O}$ | 0.06 |
*Non-zero value for the hydrogen potential at pH = 7 because [H+] = 10-7 M, not 1 M as in the standard hydrogen
electrode (SHE), and that: Ered = -0.059 V x 7 = -0.41 V
Anode: The site of water oxidation also called Oxygen Evolution Reaction (OER), often made from electrodes like iridium oxide or ruthenium oxide.
Water is oxidized to produce oxygen gas (O2), protons (H+), and electrons. These protons can then migrate through a membrane to the cathode, where they participate in the reduction reactions.
The OER is the following:
$2\text H_2 \text O \rightarrow \text{O}_2+4\text H^+ + 2\text e^-\tag{6}$
Electrolyte: Conducts ions between the electrodes. Aqueous solutions, solid oxide membranes, and polymer electrolytes are commonly used.
Facilitates the movement of ions between the cathode and anode. It can be a liquid solution or a solid polymer electrolyte.
Membrane: Separates the cathode and anode compartments, allowing selective ion transport while preventing gas crossover.
Applications and Benefits
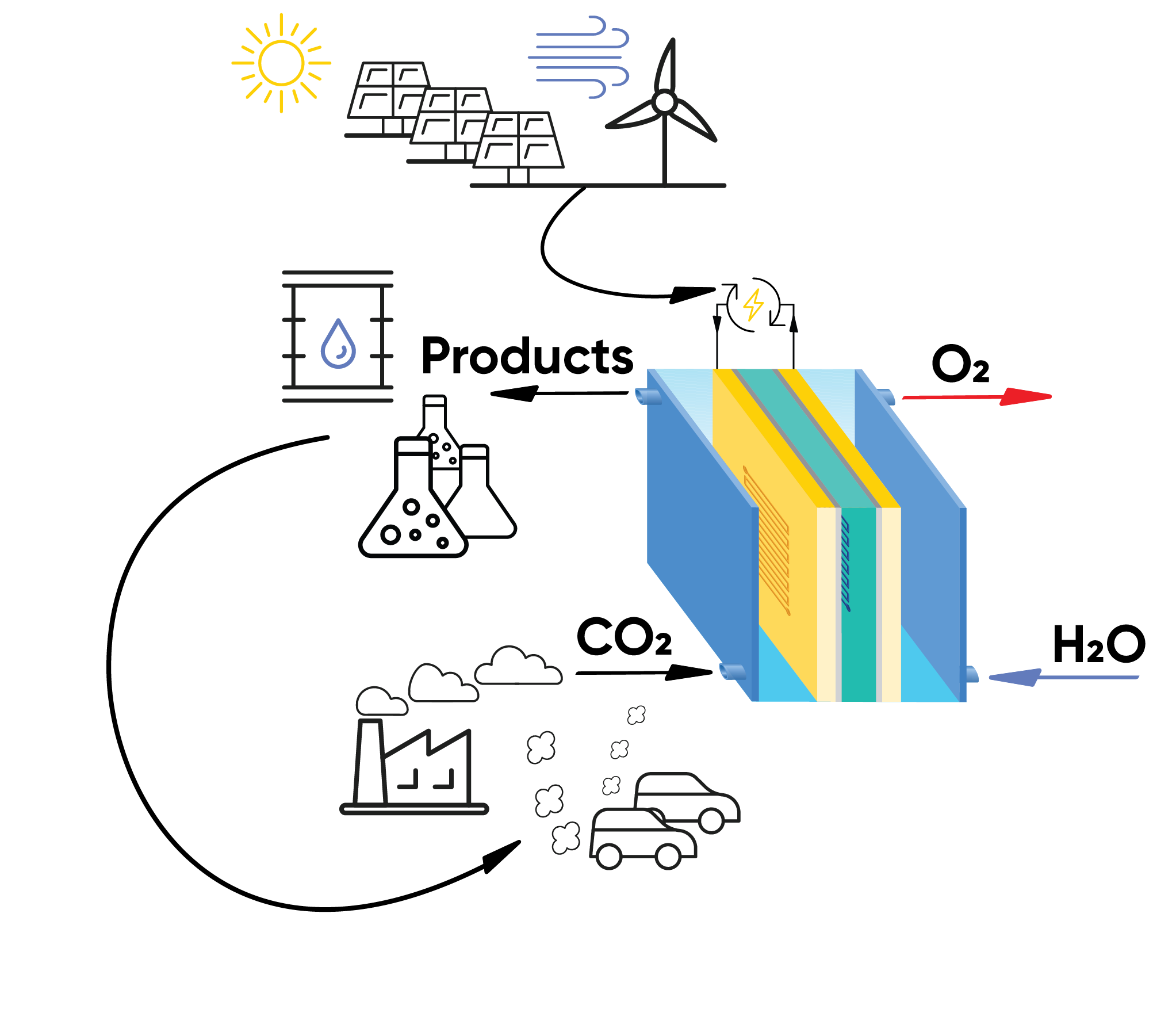
One of the main drivers for developing CO2RR technology is to lower the total amount of CO2 released into the atmosphere. By capturing and utilizing CO2, this technology has the potential to mitigate climate change.
Another benefit is a more circular carbon economy. Instead of a linear process where fossil fuels are burned, releasing CO2, CO2 can be captured and used as a feedstock, minimizing reliance on traditional fossil fuels for a more closed-loop system. Utilizing renewable electricity alongside CO2 electrolysis has the potential to produce carbon-neutral fuels like methanol or even synthetic gasoline9. Additionally, CO2 can be converted into valuable chemicals like formic acid, source of hydrogen, which can be used for energy storage or further industrial processes.
Figure 2: Scheme showing the electrochemical reduction of CO2 using renewable energy sources to produce fuels and other added value chemicals.
Products of CO2 Electrolysis
The products of CO2 electrolysis vary depending on the catalysts, cell type and operating conditions. CO2RR can include several multi-electron, multi-proton transfers8.
CO2RR produces added-value products that can be used directly or as a C1 feedstock for larger molecules. The most common product is carbon monoxide (CO), which is used as an industrial feedstock to produce various chemicals, fuels, and pharmaceuticals. Another useful product is formic acid (HCOOH). This organic acid is commonly used in the agricultural, pharmaceutical, and textile industries. HCOOH can be used directly in fuel cells for energy storage or used to produce more complex molecules10.
CO2 electrolysis can also produce alcohols like methanol (a potential fuel) and ethanol (used in biofuels and various industrial processes). However, achieving high efficiency and selectivity for these products remains a challenge. Reducing CO2 even further can produce simple hydrocarbons like methane (natural gas) and ethylene (used to make plastics). Capturing CO2 and converting it into hydrocarbons offers a potential pathway for carbon-neutral fuel alternatives but this often requires more energy input compared to other products.
Challenges
Although CO2RR is a promising technology, there are factors that need to be overcome for it to become a viable solution. Research is still needed to improve catalytic efficiency, selectivity and stability. Studies performed in aqueous solutions face issues with CO2 solubility, competition with the hydrogen evolution reaction (HER) and the electrolyte can be a source of contaminants.
Industrialization faces its own challenges because CO2 sources need to be pure, meaning that a separation step needs to be added before electrolysis. Separating pure CO2 from industrial sources for electrolysis is can also be quite difficult and costly, requiring additional energy and retrofitting existing plants.
How is CO2RR researched?
When researching and developing electrolyzers, every stage of development must be tested and optimized. This includes the individual components like the catalysts, electrodes, membranes and electrolytes, and progresses all the way to full cells and commercial stacks. Electrolysis itself is based on electrochemistry and thus potentiostats are also used to optimize output conditions.
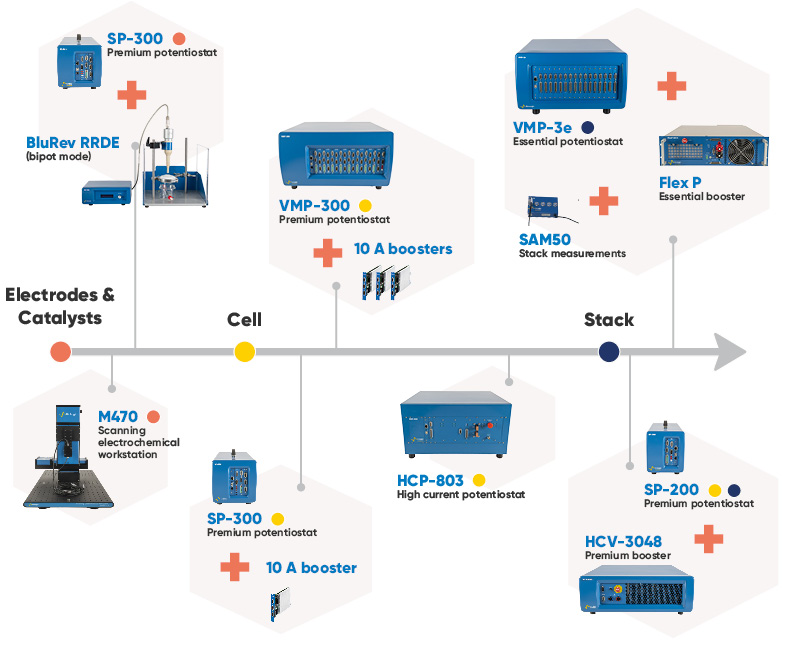
Figure 3: Range of BioLogic instruments used throughout CO2 electrolyzer research and testing.
Techniques like Rotating Disk Electrode (RDE) on the BluRev are essential for characterizing catalysts and electrodes under different electrolyte solutions and evaluating conversion under diffusion conditions. Whereas Electrochemical Impedance Spectroscopy (EIS) and the localized electrochemistry, performed on the M470, help evaluate membrane properties and optimize catalyst activity. For a high throughput screening approach, the M470 could be also used.
As research progresses to include full cells, BioLogic offers a range of modular potentiostats and boosters that can grow and change depending on current needs and number of channels desired. Premium potentiostats can be equipped with internal booster channels up to 10 A, or even 120 A with four external 30 A boosters (HCV-3048). See the article related to Premium internal boosters.
For CO2 electrolyzers with non-aqueous electrolytes, achieving high compliance voltage becomes particularly important. Non-aqueous electrolytes typically exhibit narrower electrochemical windows and operating outside this window can lead to electrolyte decomposition, decreased efficiency, and even device failure. Non-aqueous electrolytes require a power source with high compliance voltage, up to ±48 V with internal boosters or ±60 V with the FlexP 0060. This ensures the applied voltage stays within the safe operating window for the electrolyte, promoting stable and efficient CO2 conversion.
BioLogic’s EC-Lab® software simplifies data collection with the ability to modify test plans on the fly and set safety limits, which is especially important when working with the high currents required for larger membranes and stacks. Integration with gas control systems and climate chambers is also possible through OEM packages or simple Triggers within EC-Lab®. Note: a fully integrated system, where the potentiostat is coupled with a Mass Spectrometer, is available with our Spectro Inlets partnership.
For data processing and analysis, EC-Lab® offers valuable features like ZFit for modeling circuits and Levich/Koutecky-Levich analysis for kinetic studies. These comprehensive capabilities make BioLogic potentiostats a perfect choice for driving innovation in CO2 electrolyzer research.
Thanks to our EIS expertise over years, (providing accurate and reliable EIS measurement over time, is also critical for high quality research.
Conclusion
CO2 electrolysis holds promise and has the potential to create a circular carbon economy, mitigate climate change, and pave the way for a more sustainable future.
While challenges remain in catalyst development, CO2 capture and industrial integration, research is advancing. Potentiostats are instrumental in this progress, enabling researchers to optimize components and develop more efficient catalysts.
References
- Huang, C.-H. & Tan, C.-S. A Review: CO2 Utilization. Aerosol Air Qual. Res. 14, 480–499 (2014).
- Zhu, Q. Developments on CO2-utilization technologies. Clean Energy 3, 85–100 (2019).
- She, X., Wang, Y., Xu, H., Chi Edman Tsang, S. & Ping Lau, S. Challenges and Opportunities in Electrocatalytic CO2 Reduction to Chemicals and Fuels. Angewandte Chemie International Edition 61, e202211396 (2022).
- Kibria, M. G. et al. Electrochemical CO 2 Reduction into Chemical Feedstocks: From Mechanistic Electrocatalysis Models to System Design. Advanced Materials 31, 1807166 (2019).
- Etzold, B. J. M. et al. Understanding the activity transport nexus in water and CO2 electrolysis: State of the art, challenges and perspectives. Chemical Engineering Journal 424, 130501 (2021).
- Zheng, Y. et al. A review of high temperature co-electrolysis of H 2 O and CO 2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): advanced materials and technology. Chem. Soc. Rev. 46, 1427–1463 (2017).
- Overa, S., Ko, B. H., Zhao, Y. & Jiao, F. Electrochemical Approaches for CO 2 Conversion to Chemicals: A Journey toward Practical Applications. Acc. Chem. Res. 55, 638–648 (2022).
- Zhang, W. et al. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Advanced Science 5, 1700275 (2018).
- Thema, M., Bauer, F. & Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renewable and Sustainable Energy Reviews 112, 775–787 (2019).
- Chen, X., Liu, Y. & Wu, J. Sustainable production of formic acid from biomass and carbon dioxide. Molecular Catalysis 483, 110716 (2020).
0