What scanning probe electrochemistry platform is best for high throughput screening?
Latest updated: November 19, 2024Why use high throughput screening?
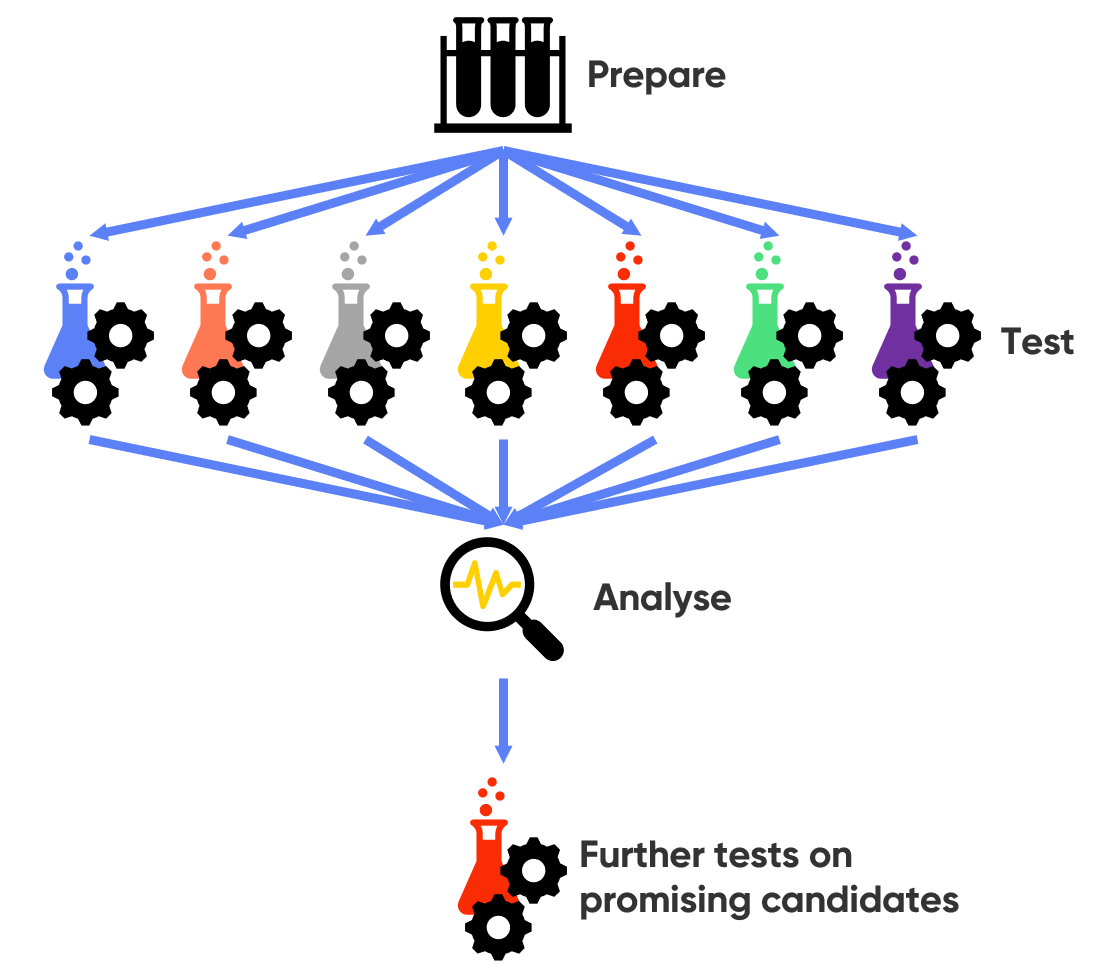
High Throughput Screening (HTS) is a screening approach for which there is growing interest for materials research, corrosion, and catalysis (including for fuel cells and electrolyzers, and CO2 electrolysis). Unlike traditional screening methods, High Throughput Screening is not carried out in an iterative manner, measuring new samples only after the study of the previous one is complete. In HTS, many samples are measured simultaneously in a single experiment, Fig. 1. While this removes the ability to inform sample design based on the study of the previous experiment, this is made up for by the substantial time savings offered. In situations where there can be almost infinite sample options, e.g., in materials and catalysis research, HTS can be the only option for measuring the number of samples available. Furthermore, the growing interest in the application of machine learning to materials research means methods like HTS, which can build up the large data sets required for high quality predictions of novel materials, are required [1].

Figure 1: The high throughput screening approach as used in electrochemical screening is illustrated.
One option to perform electrochemical HTS experiments is scanning probe electrochemistry. Scanning probe electrochemistry is the family of techniques in which a probe, in close proximity to a sample, is raster scanned across its surface to measure its local electrochemistry. It is a family which includes Scanning Electrochemical Microscopy (SECM), Scanning Droplet Cell (SDC), and Scanning Kelvin Probe (SKP), amongst others. In the next section we will look at why scanning probe electrochemistry is applied to high throughput screening.
Why is scanning probe electrochemistry used in high throughput screening?
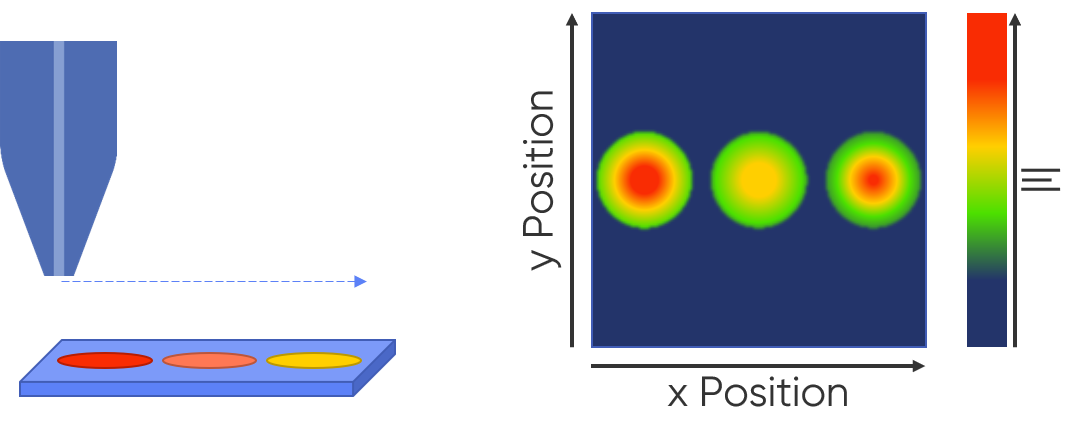
Scanning probe electrochemistry is a popular option when performing electrochemical HTS studies. An overarching benefit of scanning probe electrochemistry is that it is used for truly local measurements, which in the case of HTS means it can be used to automatically perform many individual measurements in a single experimental setup. Furthermore, these local measurements are achieved in situ, in a non-destructive manner. Regarding HTS specifically, scanning probe electrochemistry techniques offer increased reliability, and therefore comparability, between experiments than the iterative approach otherwise used for electrochemical screening. The reason for this is two-fold. First, when a sample library is simultaneously screened using scanning probe electrochemistry every sample is exposed to the same experimental conditions, including electrolyte, environment, probe, etc., reducing error from differing experimental conditions [2]. Secondly, unlike in iterative experimentation, in high throughput screening with scanning probe electrochemistry, the electrochemical cell is only built once. In iterative screening the cell must be dismantled and rebuilt between each experiment, introducing the possibility of error each time [3]. When this improved reliability and comparability is combined with the time savings offered by screening many samples at once, it is clear why there is growing interest in scanning probe electrochemistry for electrochemical screening.

Figure 2 : Using scanning probe electrochemistry, it is possible to screen multiple samples using a single experiment setup, often resulting in a map of active and inactive samples.
Which techniques are used in high throughput screening?
When scanning probe electrochemistry is used for HTS, Scanning Droplet Cell (SDC) and Scanning Electrochemical Microscopy (SECM) are the techniques most commonly used.
Scanning Droplet Cell
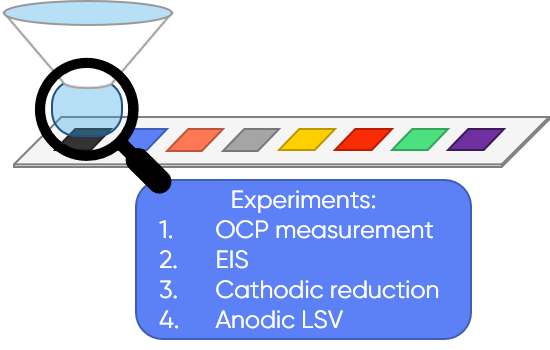
In SDC, the electrochemical cell is shrunk down to the size of a droplet confined between the sample (the working electrode) and the SDC head (containing the auxiliary electrodes). The droplet allows the electrochemical cell to measure each sample individually in a library. Because the area under the droplet forms the working electrode, each sample in the library can be measured directly, making data easy to interpret by traditional electrochemical analysis. Furthermore, this localized exposure reduces changes to the sample, which could be difficult to account for otherwise. For example, inconsistencies due to the whole sample being exposed to the electrolyte for the entire duration of the screening and those due to the whole sample being polarized for the entire screening process. SDC is typically used for HTS to automatically perform quantitative measurements of the library through local electrochemistry experiments, e.g. cyclic voltammetry, on different samples in the library. The HTS workflow used in this study is illustrated in Fig. 3.

Figure 3: The workflow used by Sur et. al. [1] to study an array of CCAs is illustrated.
Scanning Electrochemical Microscopy
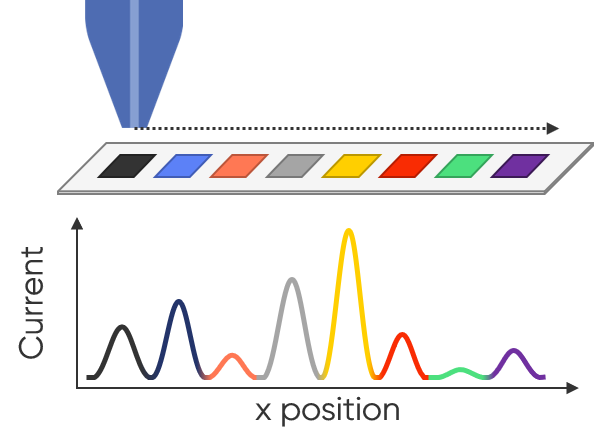
In SECM a probe, in close proximity to the sample, is used to measure the activity of the sample. Typically, the probe is biased potentiostatically to measure a current based on a redox mediator which interacts with, or is produced by, the sample. When used for HTS, SECM is usually used to perform area scans, providing a method to quickly screen a library in a qualitative manner. From this initial screening, SECM can then be used to perform quantitative follow ups on the most promising samples, using approach curves [4]. The advantage of SECM is that it can be used to measure fully conducting and fully insulating samples, and everything in between. Furthermore, the samples can remain at OCP, and disconnected from a potentiostat. This offers a lot of flexibility for which samples can be screened using SECM. This is especially useful when screening libraries for which it is difficult to make an electrical connection to the samples. Because SECM requires the probe to be biased, this can be done to interact with specific species, making SECM chemically selective, which can be particularly useful for studies of catalyst libraries, for example. The use of SECM for high throughput screening can be seen in the work by M. Black et. al. [4]. In this work SECM was used to measure combinatorial libraries of Pt-Ru fuel cell anode catalysts. By using the SECM in generator-collector mode it was possible to quickly screen arrays of catalysts to determine the most active candidates based on their increased current density. Although the result was qualitative, it then informed further quantitative studies. In Fig. 4 the use of SECM for HTS screening is illustrated.

Figure 4: Illustration demonstrating the use of SECM for HTS of catalyst samples, similar to that used by Black et. al. [4].
Other platforms
Although SDC and SECM are the most popular, other scanning probe electrochemistry techniques have also been used to a more limited extent, e.g. Scanning Vibrating Electrode Technique (SVET) and Scanning Kelvin Probe (SKP). SVET has been used to perform high throughput studies of the applicability of corrosion inhibitors on metals [5], while SKP has been used to screen photovoltaic materials [6], and thin film libraries [7].
Selecting a scanning electrochemical workstation for high throughput screening
When selecting a scanning electrochemical workstation for HTS there are a few considerations which must be made.
You should consider whether the instrument can be purchased with techniques other than the one currently of interest, so that if in the future your needs change, the instrument can be upgraded to include other techniques of interest. A modular instrument means that it can be supplied with just the necessary technique(s), while retaining the ability to grow if your needs change.
It is important to consider the instrument software, with particular attention paid to whether the necessary experiments are available and can be used in the correct workflow. For example, it is important to know whether it is possible to automate the high throughput screening, not just for area scans but also for local electrochemical experiments.
The scan range of the scanning electrochemical workstation also needs to be considered. This is important because it will determine the size of the library that can be reasonably measured by the instrument. Selecting an instrument with too small of a scan range will put a practical limitation on the combinatorial libraries which can be screened. Related to this, if screening is to be performed through an area scan the available scan velocity should also be considered, as this will affect experiment duration, something which becomes even more restrictive as the library grows in size.
Finally, when considering SECM, it is important to keep in mind that the probe to sample distance has a noticeable effect on the signal strength. As a result, if the probe to sample distance changes throughout the measurement it can lead to results which cannot be compared across a library. The instrument must maintain probe to sample distance throughout an SECM scan, even across a tilted sample. It is important, therefore, to assess which features are available to counteract sample tilt.
BioLogic’s M470 offers users a modular scanning probe electrochemistry platform which is well suited for electrochemical high throughput screening. In Table 1 the ability of the M470 to meet the requirements outlined in this section are summarized.
Table 1: The ability of the M470 to meet the considerations for selecting a scanning electrochemical workstation for high throughput screening applications.
| Scanning electrochemical workstation considerations | M470 |
|---|---|
| Is the instrument modular? | Yes, the M470 is a modular instrument, with the following techniques available: SECM, ic-SECM, LEIS, OSP, SDC, SKP, SVET. Whether it’s a single or multi-technique instrument you are after the M470 offers a wide range of techniques. For further flexibility, these can be included during the initial purchase or through later upgrade. |
| Are the necessary experiments there? | Users of the M470 software have access to scanning experiments, local electrochemistry and corrosion experiments, and local EIS measurements, in a single software. All experiments can be sequenced to automatically run an experimental protocol. |
| Is a large enough scan range offered? | The M470 offers a scan range of 110 mm in every scan direction. |
| Is the experiment time short enough? | The M470 offers a number of ways to reduce the time required to run an experiment. The scan rate can be up to 10 mm/s in every direction. In addition, the scan rate during the measurement can be set separately to the return rate. With the M470 it is also possible to use the sweep scan mode, which offers the best experiment times. |
| How does the instrument maintain constant probe to sample distance in SECM? | There are two options on the M470 to avoid changing probe to sample distance in SECM. For flat samples, typical of a combinatorial library, it is possible to perform tilt compensation during the SECM area scan, over the entire 110 mm range in x and y. For samples with noticeable background topography ic-SECM can be used. |
Conclusion
Scanning probe electrochemistry is well suited to performing electrochemical high throughput screening studies. Of particular interest for this field are SECM and SDC, which have been used for screening libraries of novel alloys, catalysts and more. When deciding to use scanning probe electrochemistry for your high throughput screening work it is important to consider the workstation which will be used. For more information on how BioLogic can meet your high throughput screening needs please contact your local representative.
References
- Sur, H. Joress, J. Hattrick-Simpers, J. R. Scully, Journal of The Electrochemical Society 170 (2023) 081507
- Vijayakumar, J. G. Mathew, A. Muzikansky, H.-N. Barad, D. Zitoun, Mater. Adv., 4 (2023) 4472-4481
- Rahmanian, S. Fuchs, M. Fichtner, H. Sören Stein, 31/12/23, Autonomous millimeter scale high throughput battery research system (Auto-MISCHBARES), https://doi.org/10.26434/chemrxiv-2024-rb9j7-v2
- Black, J. Cooper, P. McGinn, Meas. Sci. Technol. 16 (2005) 174–182
- Kallip, A.C. Bastos, M.L. Zheludkevich, M.G.S. Ferreira, Corrosion Science 52 (2010) 3146–3149
- P. Rajbhandari, A. Bikowski, J. D. Perkins, T. P. Dhakal, A. Zakutayev, Solar Energy Materials and Solar Cells 159 (2017) 219-226
- Burgstaller, M. Hafner, M. Voith, A. I. Mardare, A. W. Hassel, J. Mater. Res., 29 (2014) 148-157