Why use RDE and RRDE method to characterize electrocatalysts?
Latest updated: November 18, 2024Introduction
Fuel cell and electrolyser technologies offer the opportunity to tackle important environmental issues, whether it is for energy storage or carbon dioxide recycling. To find out more about fuel cells and electrolysers, visit the Learning Center articles: What are PEM fuel cells and electrolyzers and Electrochemical characterisation of fuel cells and electrolysers.
One key and common challenge of these technologies is to find suitable electrocatalyst to drive reactions (oxygen reduction reaction, CO2 reduction reaction…). Recently, considerable resources have been dedicated to understanding fundamental and practical applications of electrocatalysis.
Hydrodynamic voltammetry, such as RDE and RRDE methods, enables assessment of both kinetic parameters and the electrocatalyst reaction mechanism.
Note: An electrocatalyst is a species involved in electrochemical reaction that increases the rate of the reaction without being consumed. Electrocatalyst can also tune the selectivity of the reaction.
Fuel cell and electrolyser
CO2 electroreduction
With growing interest in CO2 electrolytic conversion to valuable chemicals and fuels, being able to rapidly and accurately screen electrocatalysts plays a fundamental part in the development of CO2 electrolyser.
Understanding kinetics and the reaction mechanism of CO2 electroreduction is important for tuning electrocatalytic selectivity but it remains challenging due to mass limitations associated with CO2 solubility in solution and complexity of reaction (i.e. manifold reactive intermediates)[1].
Depending on the electrochemical process of CO2 reduction reaction (CO2RR), i.e. the number of proton-coupled electron transfers, different useful chemicals can be produced such as carbon monoxide, formic acid/formate, alcohols (methanol, ethanol…), hydrocarbons (methane, ethylene…).
$\text{CO}_2 + \text{H}^+ + 2\text{e}^-\rightarrow \text{HCOO}^-$
$\text{CO}_2 + 2\text{H}^+ + 2\text{e}^-\rightarrow \text{CO} + \text{H}_2\text{O}$
$\text{CO}_2 + 6\text{H}^+ + 6\text{e}^-\rightarrow \text{CH}_3\text{OH} + \text{H}_2\text{O}$
$\text{CO}_2 + 8\text{H}^+ + 8\text{e}^-\rightarrow \text{CH}_4 + 2\text{H}_2\text{O}$
Furthermore, the hydrogen evolution reaction from water electrocatalysis is an unwelcome side reaction competing with CO2 electroreduction that needs to be controlled[2]. Generated from CO2 electroreduction, H2 (from undesired side reactions with lower selectivity), products of interest like CO or HCOO and their mixtures can be electrochemically identified on a Rotating Ring Disk Electrode (RRDE) [3].
Oxygen reduction reaction
Oxygen reduction reaction (ORR) is the reaction occurring at the cathode of the fuel cell. As platinum-based catalysts are being replaced by heterogeneous catalysts that are less expensive and less scarce, quickly finding the best catalyst among many suitable newly-design materials becomes essential.
Rotating Disk Electrode (RDE) enables assessment of kinetics of ORR while RRDE enables the study of undesired side reactions, like peroxide production, when a two-electron pathway reaction occurs instead of the preferable four electron pathway.
$\text{O}_2 + 4\text{H}^+ + 4\text{e}^-\rightarrow 2\text{H}_2\text{O}$
$\text{O}_2 + 2\text{H}^+ + 2\text{e}^-\rightarrow \text{H}_2\text{O}_2$
(in acidic medium)
Two add-ons, multiple purpose
Kinetics with RDE with Levich and Koutecky-Levich
Unlike static voltammetry, like cyclic voltammetry (CV), where stationarity could be difficult to achieve, Rotating Disk Electrode (RDE) enables steady state access easily and rapidly.
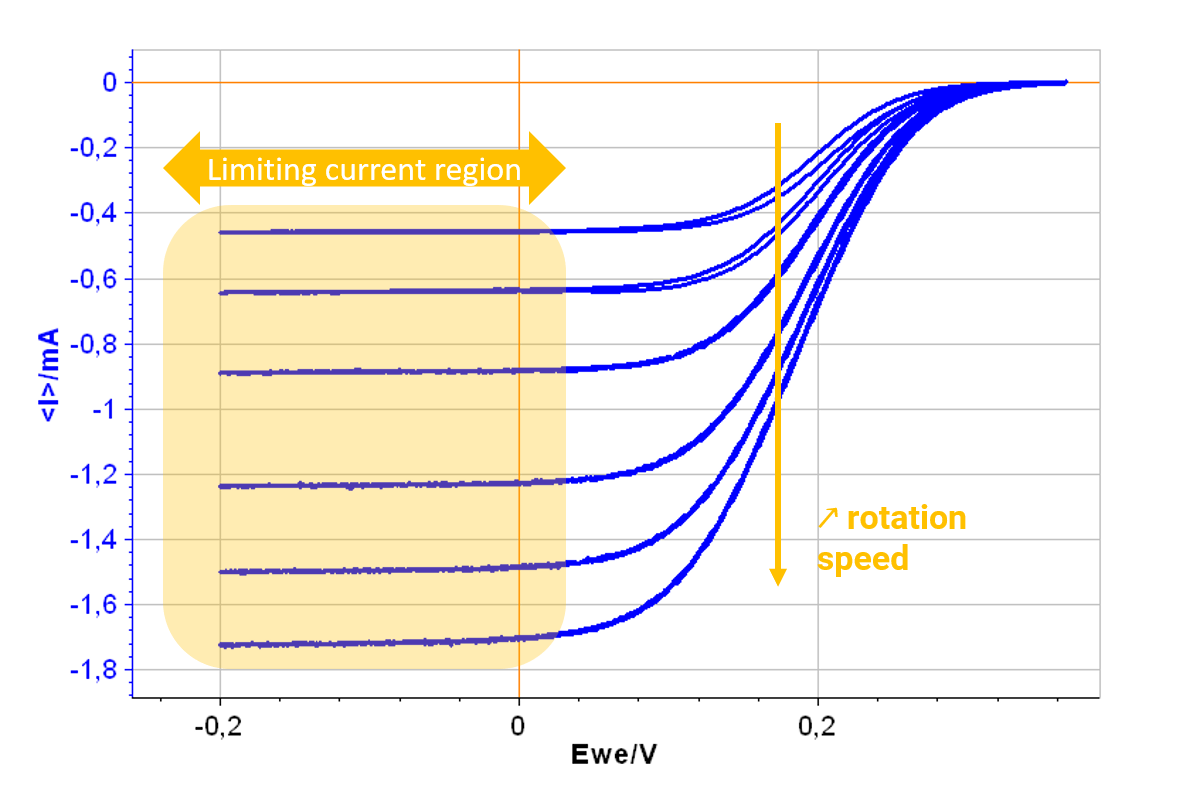
In static voltammetry, the diffusion layer thickness increases with time and there is no control over mass transfer. With a RDE, the convection of solution that is created compensates for this effect and a limiting current appears instead of a peak shaped current.

Figure 1: Results of the Levich plot experiment. The current plateaus occur at potentials for which the reaction is limited by mass transfer.
With a BluRev instrument and the dedicated Levich technique in EC-Lab®, the experiment can be programmed and performed easily all in one go. Both Levich and Koutechy-Levich analysis (available in EC-Lab®) can be performed to access kinetic parameters of interest (kinetic rate, symmetry factor) automatically.
Note: RDE can also be used to determine diffusion coefficient and can be done easily thanks to impedance spectroscopy. To find out more about this technique: .
Reaction mechanism with RRDE
Compared to RDE, adding the ring electrode allows the user to collect species produced on the disk. In more detail, with Rotating Ring Disk Electrode (RRDE), products generated at the disk are convectively transported to the ring, where they are directly detected (significantly low time delay between product generation and detection).

Figure 2: Redox reaction on a Rotating Ring Disk Electrode
While reduction is occurring on the disc, a cycling potential at the ring can directly give the identity of, and information on, the generated product (intensity of peak current, peak potential, integrated charge…).
A characteristic value that must be considered is the collection efficiency of the ring electrode: $N=- I_\text{Ring}/I_\text{Disk}$. This coefficient is characteristic of the RRDE and represents the percentage of material collected at the ring.
As for the RDE method, BluRev instruments, driven by EC-Lab software, can be used for the RRDE technique. In EC-Lab®, bipotentiostat techniques (CV-CA, CP-CA, CA-CA) are especially dedicated to RRDE investigations. They are available on multichannel potentiostats (e.g. SP-300) where two channels are synchronized to conduct two techniques simultaneously, one on each electrode. To simplify data analysis, it gives one file with all the synchronized data coming from the ring and the disk.
“CE to ground” connection mode available on BioLogic potentiostats (Premium and Essential with “e-type” channel boards) is essential to conduct accurate experiments in RRDE. It was intentionally designed to perform experiments using several working electrodes in the same electrochemical cell and avoid leakage current.
Conclusion
Understanding electrocatalysts properties is essential, whether it is for fuel cells or electrolysers. BioLogic offers a complete solution with BluRev instrument, an electrode rotator providing RDE and RRDE methods to accurately study electrochemical reactions.
| RDE | RRDE | |
| What for? | Kinetics | Reaction mechanism |
| Add-on | BluRev | BluRev |
| Potentiostat | Single channel | Multi-channel |
| Software | Compatible with EC-Lab® / controlled by EC-Lab® | Compatible with EC-Lab® / controlled by EC-Lab® |
Reference
[1] CHAUHAN, Piyush, HERRANZ, Juan, WINZELY, Maximilian, et al. Interfacial pH and Product Selectivity Measurements during CO2 Reduction on a Rotating Ring-Disk Electrode. The Journal of Physical Chemistry C, 2023, vol. 127, no 33, p. 16453-16463.
[2] GOYAL, Akansha, MARCANDALLI, Giulia, MINTS, Vladislav A., et al. Competition between CO2 reduction and hydrogen evolution on a gold electrode under well-defined mass transport conditions. Journal of the American Chemical Society, 2020, vol. 142, no 9, p. 4154-4161.
[3] ZHANG, Fen, et al. Rapid product analysis for the electroreduction of CO2 on heterogeneous and homogeneous catalysts using a rotating ring detector. Journal of The Electrochemical Society, 2020, vol. 167, no 4, p. 046517.